What is Bioreactor?
Specialized containers or setups called bioreactors are used to help bacteria or cells grow under supervision. It come in different varieties, such as photobioreactors and stirred tank bioreactors. Fermenters are another name for bioreactors, which are devices where fermentation takes place.
It is a closed vessel or system used to produce medicines, biofuels, or enzymes by cultivating bacteria, cells, or tissues under carefully monitored conditions.
Table of Contents
Design Principles
Sterility
To avoid contamination and guarantee the growth of the intended organisms, the bioreactor environment needs to be sterile.
Agitation and Mixing
In order to ensure that nutrients, oxygen, and heat are distributed uniformly throughout the reactor and to support effective cell growth, proper mixing is crucial.
Temperature Control
For metabolic activity and cell viability, the ideal temperature must be maintained.
pH Control
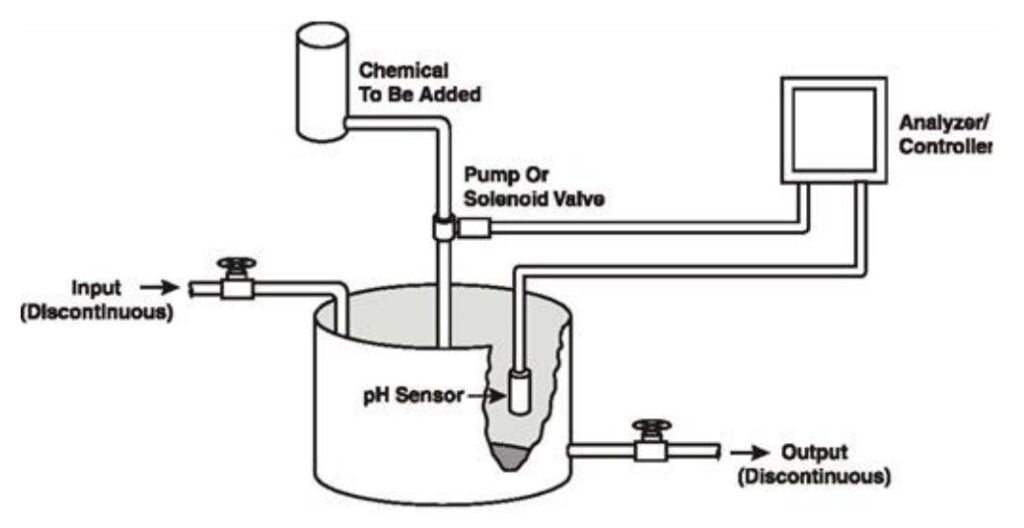
Enzyme activity and cell development depend on maintaining optimal pH levels.
Oxygen Transfer
Through aeration or sparging, an adequate supply of oxygen is made available to aerobic organisms.
Monitoring and Control
Accurate process control is made possible by constant observation of critical variables such as pH, temperature, dissolved oxygen, and cell density.
Parts
Vessel
The primary container for the organisms and culture medium.
Agitation system
Agitation system guarantees uniform distribution of nutrients and oxygen while facilitating mixing.
Aeration system
The aeration system provides the culture with oxygen.
System for controlling temperature
Preserves the ideal temperature.
pH control system
Adjusts the medium’s pH .
Monitoring and Controlling system
Key parameters are monitored and controlled by the monitoring and control system.
Sampling port
Enables the collection of samples for examination.
Ports for input and output
Used to add nutrients and remove waste.
Types
Sure! In a variety of biochemical processes, bio reactors are essential because they foster the growth and production of useful metabolites by microorganisms. The types include the following:
Continuous Stirred Tank Bio reactor (CSTR)
One of the most popular varieties is the Continuous Stirred Tank Bioreactor (CSTR). Its constant culture medium mixing ensures consistent conditions for microbial growth and product generation.
Airlift Bio reactor
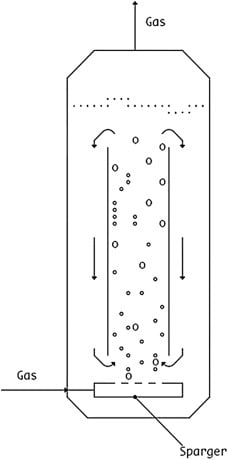
This type produces a mild mixing effect by using gas to move the medium. For species that are sensitive to shear, it is very helpful.
Bubble Column Bio reactor
In this type of bio reactor, gas bubbles rise through the liquid and are shaped like vertical columns. For aerobic fermentations, they are frequently utilized.
Fluidized Bed Bio reactor
In this arrangement, a liquid or gas flow upward suspends solid particles. It works well for gas-liquid-solid reactions and immobilized cell cultures.
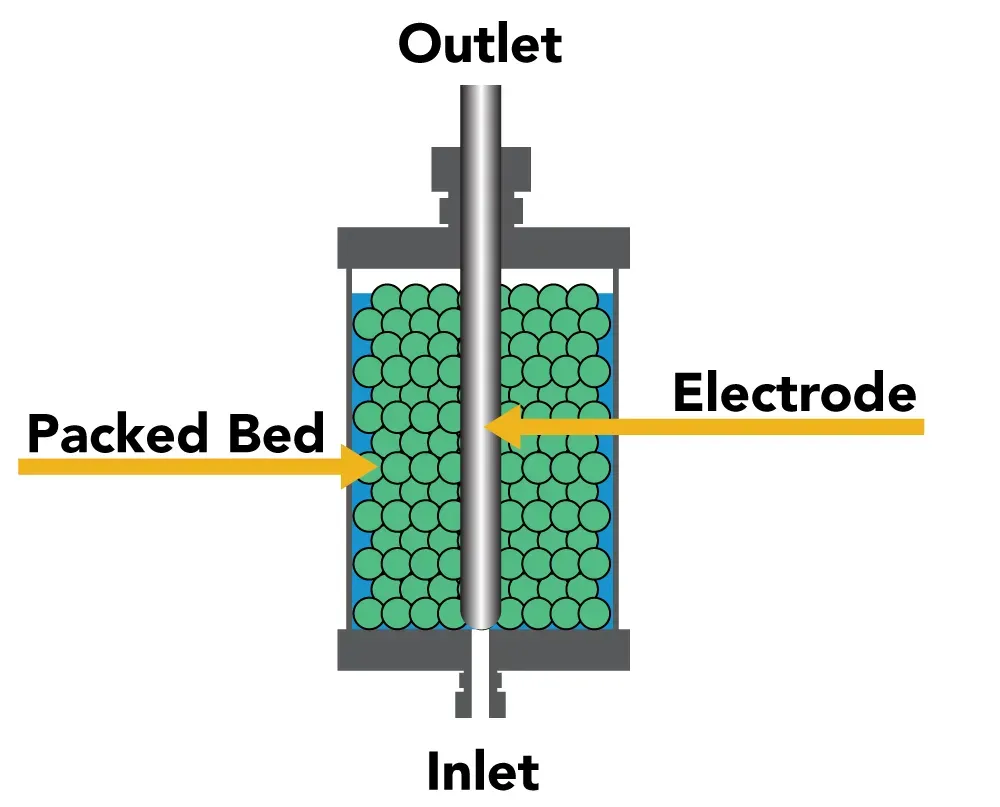
Packed Beds Bio reactor
In this instance, a packed bed of solid particles (like fibers or beads) forms. This bed allows for effective mass transfer and high cell density since the liquid flows across it.
Photobioreactor
Photobioreactors use light to drive biological reactions; they are designed for photosynthetic organisms. They are frequently employed in the culture of algae and cyanobacteria.
Applications of Bio reactor
Manufacturing of vaccines, antibiotics, hormones, and other therapeutic proteins is the pharmaceutical sector.
Production of bioethanol, biodiesel, and biogas is known as the biofuel industry.
Bread, cheese, yoghurt, and other fermented goods are produced here.
Using microorganisms to clean up contaminated surroundings is known as bioremediation.
Developing tissues and cells for use in medicine.
Limitations
Challenges associated with scaling up: It can be difficult to transfer a procedure from small-scale laboratory bioreactors to large-scale industrial bioreactors.
Maintenance of sterility: It’s crucial to keep an atmosphere sterile, and any contamination might throw things off.
High running costs: The mixing, aeration, and temperature control processes in bioreactors demand a large amount of energy.
Maintenance and cleaning: To guarantee peak performance, routine maintenance and cleaning are needed.
Process optimization : It can take a lot of time and resources to determine the ideal conditions for particular organisms and products.
In conclusion, bio reactors are essential instruments in contemporary biotechnology because they provide a regulated setting for the large-scale synthesis of a variety of goods. For efficient application and optimisation in a variety of sectors, it is essential to comprehend their design, principles, and constraints.
Frequently Asked Questions(FAQ)
What is Bioreactor?
It is a closed vessel or system used to produce medicines, biofuels, or enzymes by cultivating bacteria, cells, or tissues under carefully monitored conditions.
Define about photobioreactor ?
Photobioreactors use light to drive biological reactions; they are designed for photosynthetic organisms. They are frequently employed in the culture of algae and cyanobacteria.
What are the limitation of Bioreactor ?
The limitation of bio reactor are
1.It can be difficult to transfer a procedure from small-scale laboratory bioreactors to large-scale industrial bioreactors.
2. It’s crucial to keep an atmosphere sterile, and any contamination might throw things off.
3.It can take a lot of time and resources to determine the ideal conditions for particular organisms and products.
Related Articles