What is Sanger Sequencing?
Sanger sequencing (also called the chain-termination method) is a groundbreaking DNA sequencing technique developed by Frederick Sanger in 1977. It was the first widely used method to accurately read the order of nucleotide bases (A, T, C, G) in a DNA strand essentially, it lets scientists “decode” genes letter by letter.
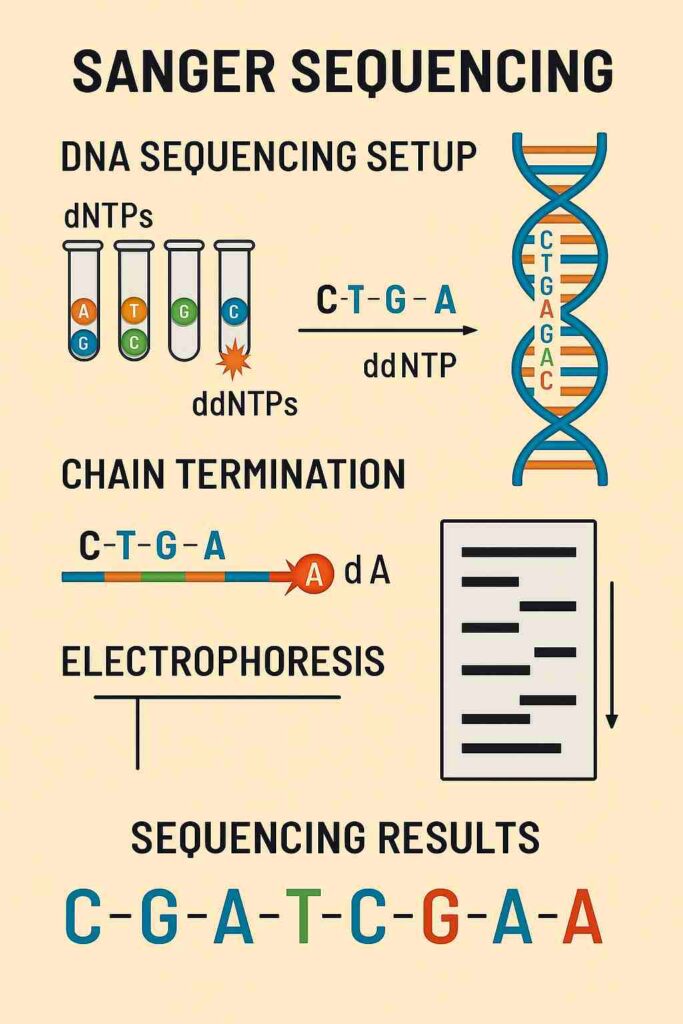
Gene sequencing is the process of determining the exact order of nucleotides (A, T, C, G) in a DNA molecule. Among the various techniques developed, Sanger sequencing, also known as the chain-termination method, stands as one of the most revolutionary breakthroughs in molecular biology. Developed by Frederick Sanger in 1977, this method became the gold standard for DNA sequencing for decades and played a crucial role in major scientific projects like the Human Genome Project.
Unlike modern high-throughput sequencing technologies, Sanger sequencing is a precise, reliable, and straightforward method that allows scientists to read DNA sequences with high accuracy. Even today, it remains widely used for validating results from newer sequencing techniques.
Summary of Sanger Sequencing
- Sanger sequencing is the original DNA decoding method that reads genetic sequences with perfect accuracy by using special chain-terminating molecules.
- It delivers reliable results in 24-48 hours, making it ideal for medical diagnoses, crime lab forensics, and double-checking genetic research findings.
- While newer technologies are faster, Sanger remains the trusted “fact-checker” of genetics because of its bulletproof precision for small DNA segments.
Table of Contents
Steps of Sanger sequencing method
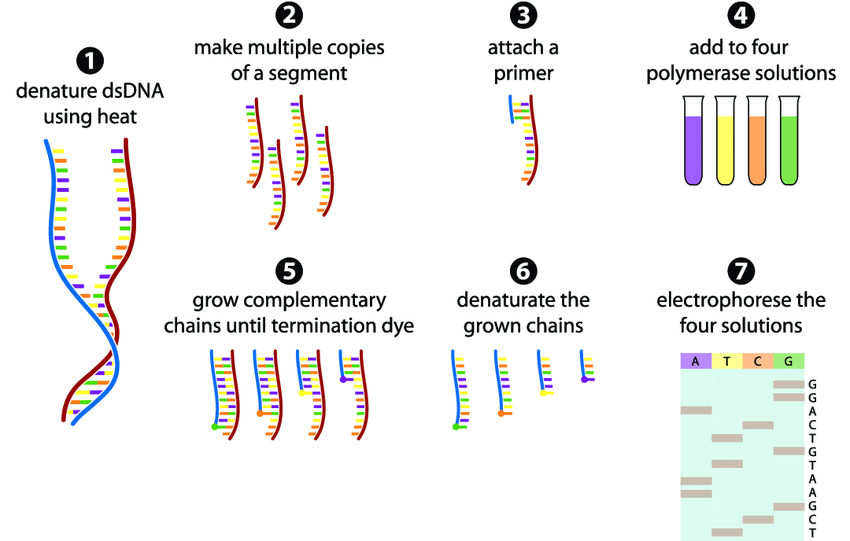
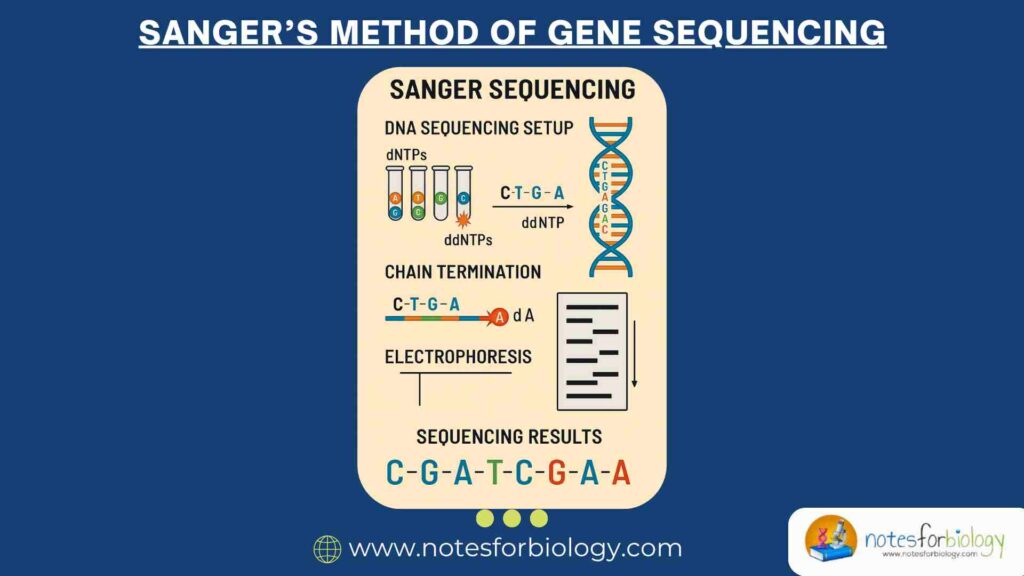
Sanger sequencing is a method used to read the order of nucleotides (A, T, C, G) in a DNA strand. Below is a simple, step-by-step explanation of the process based on your diagram:
1. Denature Double-Stranded DNA Using Heat
The process starts with double-stranded DNA (dsDNA). To separate the two strands, the DNA is heated. This breaks the hydrogen bonds between the bases, turning dsDNA into single-stranded DNA (ssDNA). This step is crucial because sequencing requires a single template strand.
2. Make Multiple Copies of a Segment
The single-stranded DNA is then copied many times using PCR (Polymerase Chain Reaction). This ensures there are enough DNA fragments to work with, improving the accuracy of sequencing.
3. Attach a Primer
A short piece of DNA called a primer is added. The primer binds to the start of the target DNA sequence, providing a starting point for DNA polymerase (the enzyme that builds new DNA strands).
4. Add to Four Polymerase Solutions
The DNA is divided into four separate tubes, each containing:
- DNA polymerase (to build new strands)
- Normal nucleotides (A, T, C, G)
- One type of special dye-labeled dideoxynucleotide (ddNTP)—either ddATP, ddTTP, ddCTP, or ddGTP
These ddNTPs act as “chain terminators” because they stop DNA synthesis when they are added.
5. Grow Complementary Chains Until Termination
DNA polymerase starts copying the template strand, adding normal nucleotides. However, when a ddNTP is randomly inserted, replication stops. This produces DNA fragments of different lengths, each ending with a fluorescently labeled ddNTP.
- Example: If the sequence is ATCG, the fragments could be:
- A (stopped at A)
- AT (stopped at T)
- ATC (stopped at C)
- ATCG (stopped at G)
6. Denature the Grown Chains
The newly synthesized DNA strands (now a mix of different lengths) are separated from the template. This prepares them for the next step electrophoresis.
7. Electrophorese the Four Solutions
The fragments are loaded into a gel or a capillary tube, and an electric current is applied. Shorter fragments move faster, while longer fragments move slower.
- Each ddNTP has a unique fluorescent color:
- A (Green)
- T (Red)
- C (Blue)
- G (Yellow/Black)
Final Output: The DNA Sequence
By detecting the order of the colored peaks, scientists can read the original DNA sequence from the shortest to the longest fragment.
Example Result:
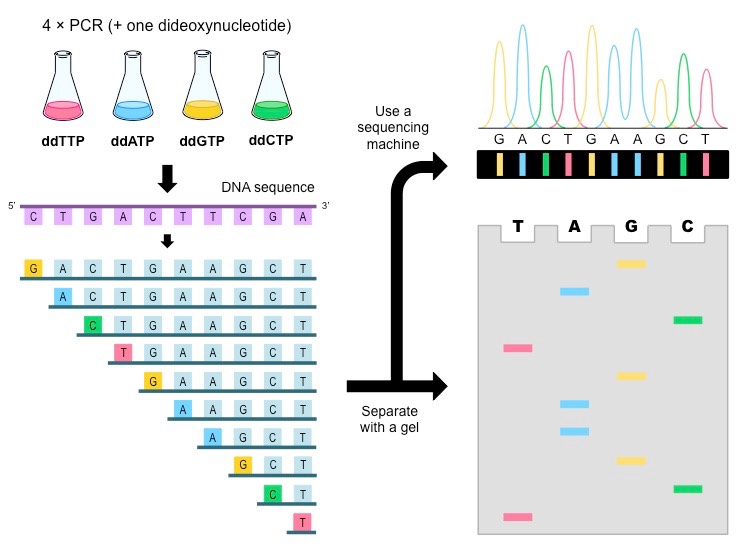
If the fragments end in:
- G (shortest)
- C
- T
- G
- T
- A
- A
- G
- C
- T (longest)
The sequence is: G → C → T → G → T → A → A → G → C → T
Why Was Sanger Sequencing Revolutionary?
Before Sanger’s method, DNA sequencing was slow and labor-intensive. Earlier techniques relied on partial digestion or RNA sequencing, which were less efficient. Sanger sequencing introduced:
- High Accuracy: Fewer errors compared to earlier methods.
- Scalability: Could be used for longer DNA strands (up to ~1000 base pairs).
- Automation Potential: Later adaptations allowed machines to perform sequencing, speeding up research.
This innovation earned Frederick Sanger his second Nobel Prize in Chemistry (his first was for sequencing insulin), making him one of only four people to win two Nobel Prizes
Applications of Sanger Sequencing
1. Medical Diagnostics & Genetic Disease Testing
Sanger sequencing plays a crucial role in identifying mutations responsible for genetic disorders. It is the preferred method for confirming diagnoses of conditions like cystic fibrosis and sickle cell anemia due to its unmatched accuracy. In cancer research, it helps verify mutations in genes like BRCA1 and BRCA2, guiding personalized treatment plans. The technique is also vital in pharmacogenomics, where it determines how patients metabolize medications based on their genetic makeup, ensuring safer and more effective drug prescriptions.
2. Microbiology & Infectious Disease Research
Scientists rely on Sanger sequencing to study pathogens and track disease outbreaks. By sequencing bacterial 16S rRNA or viral genomes, researchers can identify antibiotic-resistant strains like MRSA and monitor mutations in viruses such as HIV and SARS-CoV-2. This method provides precise data for developing targeted treatments and vaccines, making it indispensable in combating infectious diseases.
3. Forensic Science & Legal Investigations
In forensic labs, Sanger sequencing is used for DNA fingerprinting to solve crimes and establish biological relationships. It compares DNA samples from crime scenes with suspects or missing persons, providing reliable evidence for legal cases. The technique also supports wildlife forensics by identifying endangered species in illegal trade, helping authorities enforce conservation laws.
4. Agricultural & Animal Genetics
Farmers and biotechnologists use Sanger sequencing to improve crops and livestock. It verifies genetically modified organisms (GMOs) by checking inserted gene sequences, ensuring compliance with regulations. In animal breeding, it identifies genes linked to desirable traits, such as disease resistance in cattle or higher milk production, enhancing agricultural productivity.
5. Research & Biotechnology
Sanger sequencing validates results from next-generation sequencing (NGS), ensuring accuracy in genomic studies. It confirms successful gene edits in CRISPR experiments and sequences ancient DNA from fossils, offering insights into evolutionary biology. Its precision makes it a trusted tool for quality control in biotech labs, where even minor errors can have significant consequences.
6. Quality Control in Industry
Pharmaceutical companies use Sanger sequencing to verify the DNA sequences of synthetic drugs like insulin, guaranteeing product safety and efficacy. In the food industry, it detects mislabeling or contamination, such as identifying substitute species in seafood products. This application ensures compliance with health standards and protects consumers from fraud.
Why Sanger Sequencing Remains Essential
Despite advancements in high-throughput technologies, Sanger sequencing is irreplaceable for applications requiring absolute precision. Its ability to deliver accurate, reliable results for small DNA segments makes it the gold standard for clinical diagnostics, forensic analysis, and critical research. While newer methods excel in speed and scale, Sanger sequencing continues to be the definitive tool for validating genetic information.
Limitations of Sanger Sequencing
1. Short Read Length
Sanger sequencing can only analyze DNA fragments of 500-1000 base pairs at once. For longer sequences, researchers must break DNA into smaller pieces and sequence them individually, significantly increasing both time and cost. This limitation makes it inefficient for whole-genome sequencing projects.
2. Low Throughput
The method processes just one DNA fragment per reaction, unlike modern high-throughput techniques that analyze millions simultaneously. This makes Sanger sequencing impractical for large-scale genomic studies, restricting its use to targeted sequencing of specific regions.
3. High Cost for Large Projects
While economical for small applications, costs escalate quickly for extensive sequencing. Each reaction requires separate reagents and processing, making whole-genome sequencing far more expensive than with next-generation methods.
4. Time-Consuming Process
The multi-step workflow – from sample prep to electrophoresis – requires significant hands-on time. Processing multiple samples can take days, making it slower than automated NGS platforms for time-sensitive applications.
5. Difficulty Detecting Low-Frequency Mutations
The technique can’t reliably identify mutations present in less than 15-20% of cells in a sample. This limitation is problematic for cancer research or heterogeneous infections where detecting rare variants is crucial.
6. Dependence on Primer Design
Success requires perfectly designed primers that bind specifically to target DNA. Poor primer design leads to failed reactions or incomplete data, adding complexity when working with unknown genomic regions.
7.Limited Scalability
The method doesn’t easily adapt to increasing sample numbers. Scaling up requires proportionally more resources, unlike NGS where costs per sample decrease with higher throughput.
Sanger Sequencing vs. Next-Generation Sequencing (NGS)
| Feature | Sanger Sequencing | Next-Gen Sequencing (NGS) |
|---|---|---|
| Read Length | ~500-1000 bp | 50-300 bp (but massively parallel) |
| Throughput | Low (one sequence at a time) | High (millions of sequences at once) |
| Cost | Expensive per base for large genomes | Cheaper for whole genomes |
| Accuracy | Very high (~99.99%) | Slightly lower but improving |
| Best For | Small regions, validation | Whole genomes, large-scale studies |
While NGS dominates large projects, Sanger sequencing remains the gold standard for accuracy in small-scale applications.
Conclusion
Sanger sequencing may not be the flashiest or fastest DNA analysis method today, but its unmatched accuracy keeps it in every geneticist’s toolkit. Think of it like a trusted microscope while we now have high-tech scanners, sometimes you still need that precise, hands-on approach to see the clearest picture. Yes, it’s slower for big jobs and can’t handle massive datasets like newer methods, but when it comes to validating critical results, diagnosing genetic diseases, or solving crimes, scientists still turn to this classic technique.
It’s the gold standard for a reason reliable, precise, and indispensable for the details that matter most in genetics. As technology races forward, Sanger sequencing remains the careful fact-checker of the DNA world, proving that sometimes the old ways are still the best ways for getting things right.
Frequently Asked Questions (FAQs)
What sample can be Sequenced?
Accepted sample include:
Plasmids
PCR products (purified or unpurified)
Bacterial colonies
BAC DNA
What is Sanger Sequencing?
Sanger sequencing is a method to determine the exact order of nucleotides (A, T, C, G) in DNA. It uses dideoxynucleotides (ddNTPs) to stop DNA replication at random points, creating fragments of varying lengths. These fragments are then separated by size to read the sequence
How long does it take to give results?
Most labs deliver results within 24-48 hours after receiving your samples. Express options (4-8 hour turnaround) may be available for urgent projects at extra cost. Delays can occur if samples have low quality, contamination, or complex sequences (e.g., GC-rich regions).
Related Articles