What do you mean by Precipitation reactions?
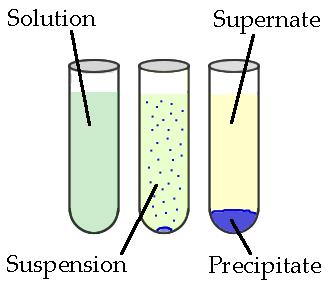
Precipitation reactions are a fundamental concept in chemistry that involve the formation of a solid precipitate when two solutions containing soluble ionic compounds are mixed. These reactions are characterized by the formation of an insoluble compound, which settles out of the solution as a visible solid. This process is driven by the formation of a more stable product, usually a less soluble compound.
Table of Contents
Understanding the Basics
Soluble Compounds: These compounds readily dissolve in a solvent, typically water, to form ions.
Insoluble Compounds: These compounds have limited solubility and tend to form a solid precipitate when their ions combine.
Precipitation Reaction: A chemical reaction where the formation of an insoluble compound (precipitate) occurs upon mixing two solutions containing soluble ionic compounds.
Driving Force Behind Precipitation
The driving force behind precipitation is the minimization of free energy in the system. When soluble ions combine to form an insoluble compound, the system reaches a lower energy state, leading to the formation of the precipitate.
Types of Precipitation Reactions
Double Displacement Reactions
In this type of reaction, two reactants exchange their ions, leading to the formation of two new products, one of which is an insoluble precipitate.
Example
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
In this reaction, silver nitrate (AgNO3) reacts with sodium chloride (NaCl) to form silver chloride (AgCl), a white precipitate, and soluble sodium nitrate (NaNO3).
Metathesis Reactions
Similar to double displacement reactions, metathesis reactions involve the exchange of ions between two reactants. However, the focus here is on the formation of a precipitate.
Example
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq)
Barium chloride (BaCl2) reacts with sodium sulfate (Na2SO4) to produce barium sulfate (BaSO4), a white precipitate, and soluble sodium chloride (NaCl).
Predicting Precipitation Reactions
To predict whether a precipitation reaction will occur, we use the solubility rules, which are guidelines that indicate the solubility of various ionic compounds in water. Some important solubility rules include:
- All Group 1 (alkali metals) cations and ammonium (NH4+) are soluble.
- All nitrate (NO3-) and acetate (CH3COO-) anions are soluble.
- Most chloride (Cl-), bromide (Br-), and iodide (I-) anions are soluble, except for those containing silver (Ag+), lead (Pb2+), and mercury(I) (Hg2+).
- Most sulfate (SO42-) anions are soluble, except for those containing barium (Ba2+), strontium (Sr2+), calcium (Ca2+), and lead (Pb2+).
- Most carbonate (CO32-), phosphate (PO43-), and hydroxide (OH-) anions are insoluble, except for those containing Group 1 cations and ammonium.
By applying these rules, we can determine whether the products formed in a potential reaction will be soluble or insoluble.
Importance and Applications of Precipitation Reactions
Precipitation reactions play a crucial role in various fields, including:
Analytical Chemistry
Precipitation reactions are extensively used for qualitative analysis, where the presence or absence of specific ions in a solution is identified based on the formation of characteristic precipitates. For example, the presence of chloride ions can be confirmed by adding silver nitrate, which forms a white precipitate of silver chloride.
Quantitative Analysis
Precipitation reactions are also used in gravimetric analysis, a technique for determining the amount of a specific substance in a sample. This involves forming a precipitate of the desired analyte and weighing it after drying.
Environmental Chemistry

Precipitation reactions are essential for removing pollutants from wastewater. For example, heavy metals like lead and mercury can be removed by precipitation using reagents like sulfide ions.
Industrial Processes
Precipitation reactions are employed in various industrial processes, such as:
Production of pigments: Pigments like titanium dioxide (TiO2) are synthesized through precipitation reactions.
Production of fertilizers: Insoluble phosphate compounds are formed by precipitation reactions to create fertilizers.
Production of soaps and detergents: Precipitation reactions are used in the production of soaps and detergents to remove impurities and create desired products.
Biological Processes
Precipitation reactions occur in biological systems, playing a role in bone formation and the regulation of mineral levels in the body.
Conclusion
Precipitation reactions are an integral part of chemistry, finding applications in various fields. Understanding the concepts of solubility, precipitation, and the driving forces behind these reactions is crucial for interpreting and predicting chemical behavior. The ability to apply these principles enables us to perform qualitative and quantitative analyses, purify substances, and develop innovative solutions for environmental and industrial challenges.
Frequently Asked Questions(FAQ)
Why chemical reaction test is performed?
Chemical testing may be done for a number of reasons, including: Ascertain whether or not a specification, rule, or contract’s requirements are being satisfied. Determine whether a program for developing new products is on track: Provide evidence of notion. Provide evidence of a proposed patent’s usefulness.
What in qualitative analysis in chemistry test?
Finding non-numerical information about a chemical species, a reaction, etc. is known as qualitative analysis. A couple of examples would be witnessing a reaction cause a change in hue or witnessing gas popping up out of solution.
Related Articles