Pre-steady-state kinetics is an essential field of research in enzyme catalysis that focuses on the initial stage of enzyme reactions before reaching a steady state. Gaining knowledge of pre-steady-state kinetics helps researchers better understand the number of active sites, the mechanisms of enzyme action, and the rate constants related to enzymatic reactions. This section will cover important methods in pre-steady-state kinetics, active site identification, rate constants, and the behavior of enzymes under limiting circumstances.
Table of Contents
Key Techniques in Pre-Steady-State Kinetics
Rapid Mixing
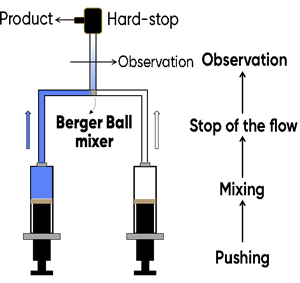
Rapid mixing techniques are used to capture the reaction’s transient states and swiftly start enzyme reactions. Researchers can virtually immediately see the first shifts in substrate concentration and product formation using this technique. The two main techniques for quick mixing are as follows:
Continuous Flow: In this technique, enzyme and substrate solutions are continuously poured into a mixing chamber, where they react nearly instantly.
Stopped-Flow Technique: This method uses spectroscopic techniques to analyze the reaction progress by quickly mixing two solutions in a chamber and stopping the reaction at predefined intervals. This method works especially well for researching quick reactions.
Relaxation Techniques
In relaxation techniques, an enzyme-substrate system is perturbed, and its equilibrium is observed to be restored. These techniques fall into the following categories:
Temperature Jump (T-jump): Rapid temperature changes in the reaction mixture can cause changes in enzyme conformation or substrate binding, allowing for the observation of relaxation kinetics.
Pressure Jump (P-jump): Like T-jump, this method uses pressure changes to examine how they affect enzyme kinetics and activity.
pH Jump: A rapid pH change is used to disrupt the ionization state of an enzyme or substrate, and the relaxation back to equilibrium is monitored.
Determination of Active Sites
The number of active sites on an enzyme is critical for understanding its catalytic efficiency. Several methods can be used to determine the number of active sites:
Enzyme Inhibition Studies: This method uses inhibitors that block the enzyme’s active site. By measuring how the enzyme activity changes when the inhibitor is added, we can estimate the number of active sites on the enzyme.
Binding Studies: Techniques like FRET and SPR help study how substrates or inhibitors bind to the enzyme, which can give information about how many active sites the enzyme has.
Ligand Binding Studies: This includes methods like:
- Spectroscopic Techniques: These measure changes in light (absorption or fluorescence) when ligands bind to the enzyme. This can help determine how many active sites are present.
- Equilibrium Dialysis: In this method, the enzyme is placed in a dialysis bag, and the amount of ligand bound to it is measured. This can also show the number of active sites.
Determination of Rate Constants
Rate constants are fundamental parameters in enzyme kinetics that describe the speed of enzymatic reactions. In pre-steady-state kinetics, rate constants can be determined using the following approaches:
Initial Rate Measurements: By measuring the initial rates of product formation at various substrate concentrations, the Michaelis-Menten equation can be applied to extract the rate constants ( k_{cat} ) (turnover number) and ( K_m ) (Michaelis constant).
Transient Kinetics: Using rapid mixing and stopped-flow techniques, researchers can observe the formation of enzyme-substrate complexes and the subsequent conversion to products, allowing for the calculation of specific rate constants for each step in the reaction mechanism.
Enzyme Kinetics at Limiting Conditions
Dilute Substrates
When substrate concentrations are extremely low, the kinetics of enzyme reactions can deviate from classic Michaelis-Menten behavior. At these low concentrations, enzyme-substrate interactions may be influenced by diffusion constraints and substrate availability. Understanding these conditions is critical for accurately modeling enzyme kinetics in physiological and industrial applications.
Solid Substrates
Enzymes that act on solid substrates present unique kinetic challenges. The enzyme’s ability to access the surface of the substrate may limit the rate of reaction. Surface area, porosity, and the solid matrix’s composition can all have a significant impact on enzyme activity and kinetics. These interactions can be studied using techniques such as enzyme immobilization on solid supports.
Enzyme Activity at Interfaces
Enzyme activity at interfaces, such as oil-water or air-water interfaces, is a critical area of investigation, particularly for enzymes involved in bioremediation or biosurfactant production. The kinetics of these reactions can be influenced by factors such as interfacial tension, enzyme orientation, and substrate partitioning among phases. Understanding enzyme behavior at interfaces is essential for optimizing reactions in biphasic systems.
Conclusion
Pre-steady-state kinetics is essential for understanding enzyme action mechanisms, determining active sites, and calculating rate constants. By employing techniques such as rapid mixing, stopped-flow, and relaxation, researchers can delve deeply into the dynamics of enzyme reactions. Furthermore, understanding pre-steady-state kinetics under limiting conditions—such as dilute substrates, solid substrates, and interface activity—is critical for applications in biochemistry, biotechnology, and enzymology. This knowledge enhances our ability to manipulate enzyme reactions for various industrial applications.
Frequently Asked Question (FAQ)
What is the significance of studying pre-steady-state kinetics in enzyme reactions?
Pre-steady-state kinetics helps us understand the early stages of enzyme reactions, such as substrate binding and intermediate production. This knowledge is critical for determining active sites, calculating rate constants, and optimizing enzyme performance in fields such as drug design and biotechnology.
How do limiting conditions affect enzyme kinetics?
Limiting conditions, such as low substrate concentrations, can cause deviations from Michaelis-Menten kinetics due to diffusion restrictions. For solid substrates, the enzyme’s access to the substrate surface may limit the reaction rate, reducing overall activity. Understanding these conditions is critical for simulating enzyme behavior in practical applications.
How can the number of active sites on an enzyme be determined?
The number of active sites can be determined through enzyme inhibition studies, in which inhibitors indicate the degree of activity loss. Binding studies using techniques such as fluorescence resonance energy transfer (FRET) or surface plasmon resonance (SPR) can also quantify active sites by examining enzyme-substrate interactions.