What is the Nitrogen Cycle ?
The movement of nitrogen between the atmosphere, biosphere, and geosphere in different forms is called the Nitrogen Cycle. It involves several processes such as nitrogen fixation, nitrification, denitrification, decay, and putrefaction. Nitrogen gas exists in both organic and inorganic forms. Organic nitrogen exists in living organisms, and they get passed through the food chain by the consumption of other living organisms. Inorganic forms of nitrogen are found in abundance in the atmosphere. This nitrogen is made available to plants by symbiotic bacteria which can convert the inert nitrogen into a usable form – such as nitrites and nitrates. Nitrogen undergoes various types of transformation to maintain a balance in the ecosystem. Furthermore, this process extends to various biomes, with the marine nitrogen cycle being one of the most complicated biogeochemical cycles.
Table of Contents
- It may also be considered as the movement of nitrogen through the food chain from simple inorganic compounds, mainly ammonia, to complex organic compounds.
- This complex cycle involves bacteria, plants, and animals.
- All organisms can convert ammonia (NH3) to organic nitrogen compounds that are compounds containing C–N bonds. However, only a few microorganisms can synthesize ammonia from nitrogen gas (N2).
- Although N2 gas makes up about 80% of the earth’s atmosphere, it is a chemically unreactive compound and thus needs to be changed in order to be utilized by living beings.
- Within the biosphere, there is a balance between total inorganic and total organic forms of nitrogen.
- The conversion of organic to inorganic nitrogen comes about through catabolism, de-nitrification, and decay.
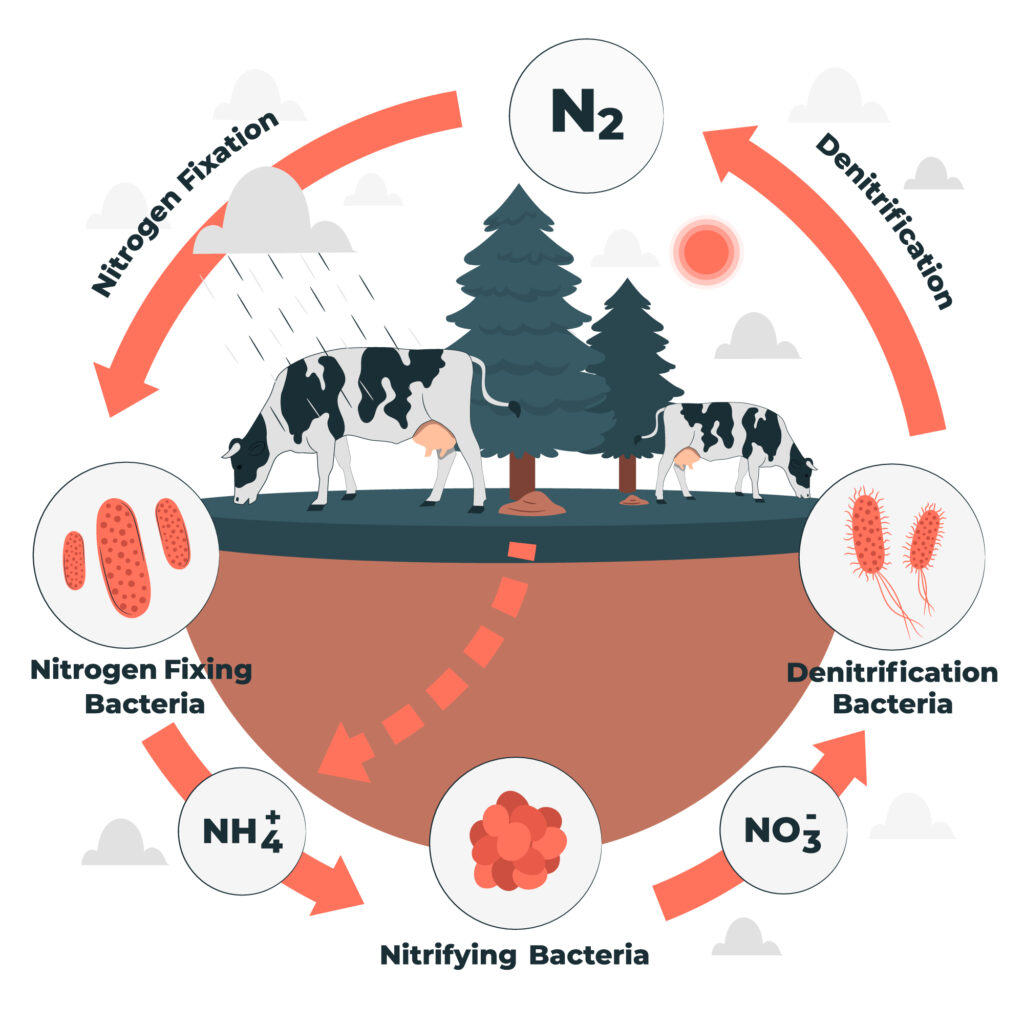
The nitrogen Cycle Diagram
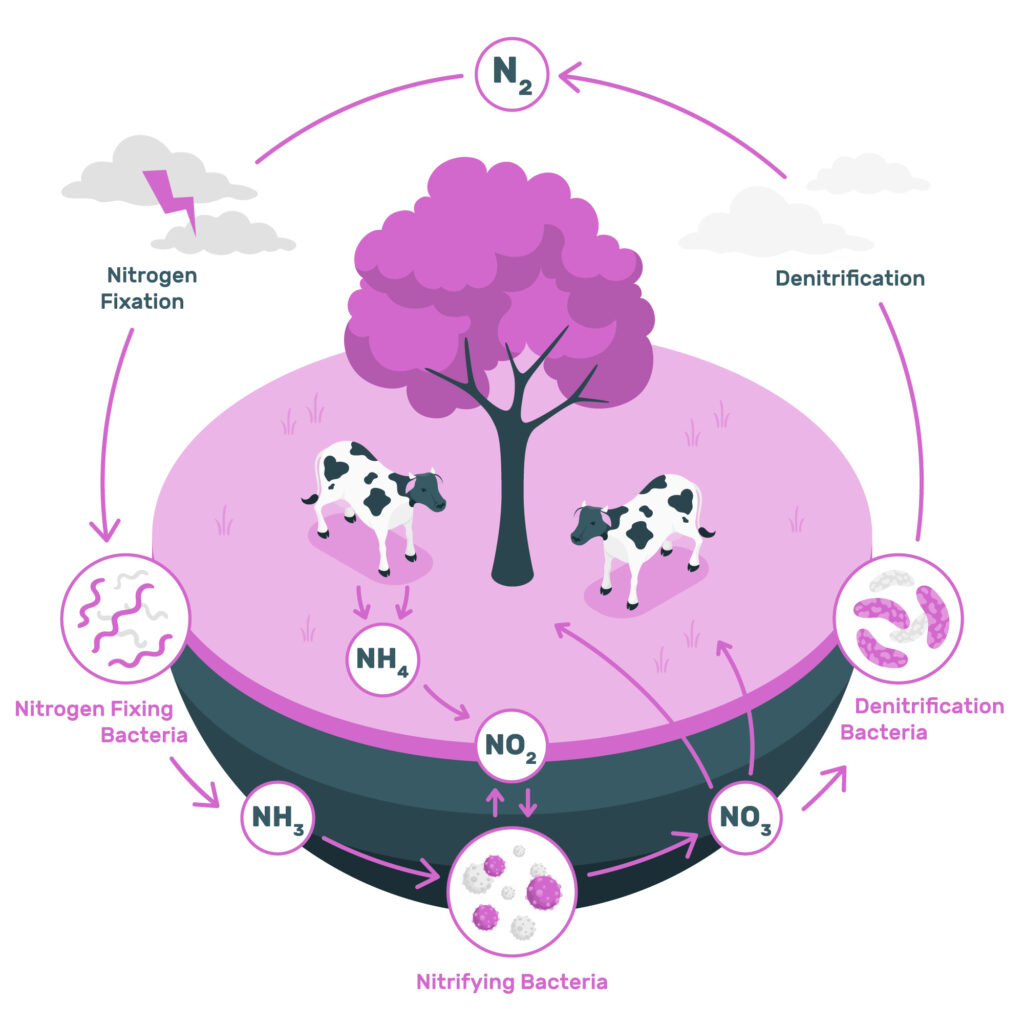
Stages of Nitrogen Cycle
The Nitrogen Cycle Process consists of the following steps:
- Nitrogen fixation
- Nitrification
- Assimilation
- Ammonification
- Denitrification
1. Nitrogen Fixation
Nitrogen Fixation is a chemical process by which molecular nitrogen, with a strong triple covalent bond, in the air is converted into ammonia or related nitrogenous compounds, typically in soil or aquatic systems but also in industry.
Process of Nitrogen Fixation
- It is the initial step of the nitrogen cycle. Here, Atmospheric nitrogen (N2) which is primarily available in an inert form, is converted into the usable form -ammonia (NH3).
- During the process of Nitrogen fixation, the inert form of nitrogen gas is deposited into soils from the atmosphere and surface waters, mainly through precipitation. Later, the nitrogen undergoes a set of changes, in which two nitrogen atoms get separated and combine with hydrogen to form ammonia (NH4+).
- The entire process of Nitrogen fixation is completed by symbiotic bacteria which are known as Diazotrophs. Azotobacter and Rhizobium also have a major role in this process. These bacteria consist of a nitrogenase enzyme that has the capability to combine gaseous nitrogen with hydrogen to form ammonia.
- Nitrogen fixation can occur either by atmospheric fixation- which involves lightening or industrial fixation by manufacturing ammonia under high temperature and pressure conditions. This can also be fixed through man-made processes, primarily industrial processes that create ammonia and nitrogen-rich fertilizers.
- Reaction: N2 + 3 H2 –> <– 2 NH3
- This process is carried out by the nitrogenase complex, which consists of a reductase and an iron–molybdenum-containing nitrogenase.
- At least 16 ATP molecules are hydrolyzed to form two molecules of ammonia.
- Leghemoglobin is used to protect the nitrogenase in the Rhizobium from inactivation by O2.
Types of Nitrogen Fixation
- Atmospheric fixation: A natural phenomenon where the energy of lightning breaks the nitrogen into nitrogen oxides and is then used by plants.
- Industrial nitrogen fixation: This is a man-made alternative that aids in nitrogen fixation by the use of ammonia. Ammonia is produced by the direct combination of nitrogen and hydrogen and later, it is converted into various fertilizsers such as urea.
- Biological nitrogen fixation: We already know that nitrogen is not usable directly from the air for plants and animals. Bacteria like Rhizobium and blue-green algae transform the unusable form of nitrogen into other compounds that are more readily usable. These nitrogen compounds get fixed in the soil by these microbes.
2. Nitrification
Nitrification is the biological oxidation of ammonia to nitrite followed by the oxidation of the nitrite to nitrate occurring through separate organisms or direct ammonia oxidation to nitrate in comammox bacteria. The transformation of ammonia to nitrite is usually the rate-limiting step of nitrification.
In this process, the ammonia is converted into nitrate by the presence of bacteria in the soil. Nitrites are formed by the oxidation of Ammonia with the help of Nitrosomonas bacterium species. Later, the produced nitrites are converted into nitrates by Nitrobacter. This conversion is very important as ammonia gas is toxic for plants.
The reaction involved in the process of Nitrification is as follows:
2NH4+ + 3O2 → 2NO2– + 4H+ + 2H2O
2NO2– + O2 → 2NO3–
Optimum conditions for Nitrification:
- Adequate aeration
- Optimum temperature 25 – 35oC
- Adequate soil moisture
- Adequate exchangeable bases – particularly Calcium (Ca)
- NPK availability
- Requires a low C/N ratio
3. Assimilation
Nitrogen Assimilation is the formation of organic nitrogen compounds like amino acids from inorganic nitrogen compounds present in the environment. Organisms like plants, fungi, and certain bacteria that can fix nitrogen gas (N2) depend on the ability to assimilate nitrate or ammonia for their needs. Other organisms, like animals, depend entirely on organic nitrogen from their food. The next step in the nitrogen cycle is the assimilation of inorganic nitrogen, into organic nitrogen-containing compounds. Primary producers plants take in the nitrogen compounds from the soil with the help of their roots, which are available in the form of ammonia, nitrite ions, nitrate ions, or ammonium ions and are used in the formation of the plant and animal proteins. This way, it enters the food web when the primary consumers eat the plants. All organisms assimilate ammonia via two main reactions catalyzed by glutamate dehydrogenase and glutamine synthetase giving rise to the amino acids glutamate (Glu) and glutamine (Gln), respectively. The amino nitrogen in Glu and the amide nitrogen in Gln are then used in further biosynthetic reactions to give rise to other compounds.
Glutamate dehydrogenase
Glutamate dehydrogenase catalyzes the reductive amination of the citric acid cycle intermediate α-ketoglutarate. Although the reaction is reversible, the reductant used in the biosynthetic reaction is NADPH. This enzyme is also involved in the catabolism of amino acids.
Glutamine synthetase
Glutamine synthetase catalyzes the incorporation of ammonia into glutamine, deriving energy from the hydrolysis of ATP. This enzyme is named a synthetase, rather than a synthase because the reaction couples bond formation with the hydrolysis of ATP. In contrast, a synthase does not require ATP. Plants take up these forms of nitrogen through their roots and incorporate them into plant proteins and nucleic acids. Animals are then able to utilize nitrogen from the plant tissues.
4. Ammonification
Ammonification is the primary process that converts reduced organic nitrogen (R–NH2) to reduced inorganic nitrogen (NH4+) through the action of microorganisms. When an organism excretes waste or dies, the nitrogen in its tissues is in the form of organic nitrogen (e.g. amino acids, DNA). Various fungi and prokaryotes then decompose the tissue and release inorganic nitrogen back into the ecosystem as ammonia in the process known as ammonification. The ammonia then becomes available for uptake by plants and other microorganisms for growth. Many different soil organisms are involved in the ammonification process including bacteria and fungi. These soil organisms break down organic matter using the carbon and energy produced, but nitrogen is released. Ammonia is also released by the process of excretion into the environment and is then available for either nitrification or assimilation.
5. Denitrification
Denitrification is a microbially facilitated process where nitrate is reduced and ultimately produces molecular nitrogen through a series of intermediate gaseous nitrogen oxide products. Denitrification is the process in which the nitrogen compounds make their way back into the atmosphere by converting nitrate (NO3-) into gaseous nitrogen (N). This process of the nitrogen cycle is the final stage and occurs in the absence of oxygen. Denitrification is carried out by the denitrifying bacterial species- Clostridium and Pseudomonas, which will process nitrate to gain oxygen and give out free nitrogen gas as a byproduct.
Conditions favoring denitrification:
- Lack of adequate O2
- Requires energy source of oxidizable organic matter for bacteria
- Warm, slightly acidic soils.
Importance of Nitrogen Cycle
The importance of the nitrogen cycle are as follows:
- Helps plants to synthesize chlorophyll from the nitrogen compounds.
- Helps in converting inert nitrogen gas into a usable form for the plants through the biochemical process.
- In the process of ammonification, the bacteria help in decomposing the animal and plant matter, which indirectly helps to clean up the environment.
- Nitrates and nitrites are released into the soil, which helps in enriching the soil with the necessary nutrients required for cultivation.
- Nitrogen is an integral component of the cell and it forms many crucial compounds and important biomolecules.
Frequently Asked Questions
What is the Nitrogen Cycle ?
The movement of nitrogen between the atmosphere, biosphere, and geosphere in different forms is called the Nitrogen Cycle. It involves several processes such as nitrogen fixation, nitrification, denitrification, decay, and putrefaction. Nitrogen gas exists in both organic and inorganic forms.
Why is Nitrogen important for life?
Nitrogen is important for life because the amino acids contain nitrogen and form building blocks that make up various components of the human body such as hair, tissues, and muscles.
What are the stages of the Nitrogen Cycle?
The stages of the Nitrogen Cycle are Nitrogen fixation, Nitrification, Assimilation, Ammonification, and Denitrification.
What is the Nitrogen Fixation?
Nitrogen Fixation is a chemical process by which molecular nitrogen, with a strong triple covalent bond, in the air is converted into ammonia or related nitrogenous compounds, typically in soil or aquatic systems but also in industry.
What are the types of Nitrogen Fixation?
The types of Nitrogen Fixation are Atmospheric fixation, Industrial nitrogen fixation, and Biological nitrogen fixation.
What is the Nitrification?
Nitrification is the biological oxidation of ammonia to nitrite followed by the oxidation of the nitrite to nitrate occurring through separate organisms or direct ammonia oxidation to nitrate in comammox bacteria.
What is the Assimilation in Nitrogen Cycle?
Nitrogen Assimilation is the formation of organic nitrogen compounds like amino acids from inorganic nitrogen compounds present in the environment. Organisms like plants, fungi, and certain bacteria that can fix nitrogen gas (N2) depend on the ability to assimilate nitrate or ammonia for their needs.