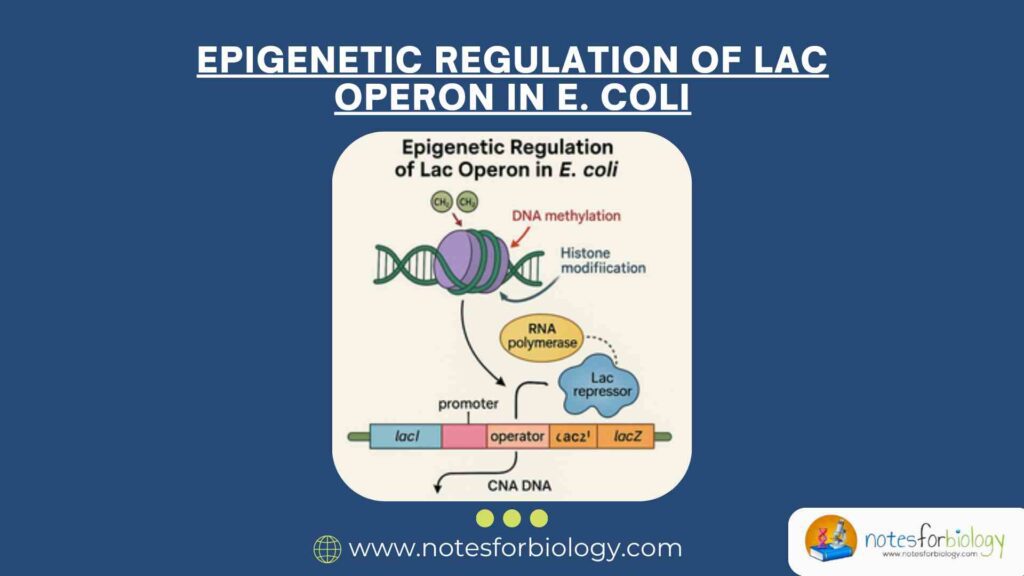
The lac operon in Escherichia coli is one of the most studied systems in molecular biology for understanding gene regulation. Discovered by François Jacob and Jacques Monod in the early 1960s, it has since become a cornerstone in the field of prokaryotic gene expression. The lac operon system illustrates how bacterial cells can adapt to environmental changes by regulating gene expression in response to the availability of nutrients.
While prokaryotic gene regulation traditionally focuses on transcription factors and regulatory sequences, recent studies suggest that epigenetic-like mechanisms, although distinct from those in eukaryotes, also play a role in bacterial gene expression. This document explores the classical model of the lac operon and investigates the emerging concept of epigenetic regulation in E. coli, with a focus on DNA methylation and nucleoid-associated proteins.
Summary of Lac Operon in E.coli
- The lac operon helps E. coli control lactose use depending on the environment.
- DNA methylation and special proteins (NAPs) can affect how the lac operon works by changing DNA shape and access.
- These changes let some bacteria act differently in the same conditions, helping them survive tough situations.
Table of Contents
Overview of the Lac Operon
Structure and Components
The lac operon consists of three structural genes: lacZ, lacY, and lacA, which code for β-galactosidase, lactose permease, and thiogalactoside transacetylase, respectively. These genes are transcribed as a single polycistronic mRNA from a common promoter (Plac). Upstream of the structural genes are regulatory elements, including:
- Promoter (Plac): Initiation site for RNA polymerase binding.
- Operator (O): DNA sequence where the lac repressor binds.
- CAP Binding Site (CBS): Site upstream of the promoter where the catabolite activator protein (CAP) binds in the presence of cyclic AMP (cAMP).
- Classical Regulation Mechanisms
In the absence of lactose, the lac repressor, encoded by the lacI gene, binds to the operator, preventing transcription by blocking RNA polymerase access to the promoter. When lactose is present, it is converted into allolactose, which binds to the repressor, causing a conformational change that releases it from the operator, allowing transcription to proceed. Additionally, when glucose is scarce, the intracellular concentration of cAMP increases, facilitating the binding of the cAMP-CAP complex to the CAP binding site, enhancing RNA polymerase affinity for the promoter.
Prokaryotic Epigenetic Mechanisms
Definition of Epigenetics in Prokaryotes
Epigenetics typically refers to heritable changes in gene expression that do not involve alterations in the DNA sequence. In eukaryotes, this includes DNA methylation, histone modification, and non-coding RNAs. Although prokaryotes lack histones and complex chromatin structures, they exhibit epigenetic-like mechanisms such as DNA methylation and regulation by nucleoid-associated proteins (NAPs).
DNA Methylation in Bacteria

In E. coli, DNA methylation predominantly occurs at adenine residues within GATC sequences, mediated by DNA adenine methyltransferase (Dam). This methylation can influence DNA-protein interactions, replication, and gene expression. Methylation status is dynamically maintained and can be inherited during cell division.
Nucleoid-Associated Proteins (NAPs)
NAPs, such as H-NS, Fis, IHF, and HU, play crucial roles in structuring the bacterial nucleoid and regulating gene expression by modulating DNA topology and accessibility. These proteins can act as transcriptional repressors or activators and contribute to the regulation of operons like lac.
Epigenetic Regulation of the Lac Operon
Role of DNA Methylation
Although the lac operon itself does not contain high densities of GATC sequences, methylation at nearby sites can influence its regulation indirectly. Methylation affects the binding affinity of regulatory proteins, such as the lac repressor and CAP, by altering the DNA conformation or competitive binding dynamics. Changes in methylation patterns can thereby modulate operon expression in response to environmental stimuli.
Recent studies have shown that Dam methylation plays a role in phase variation and gene silencing in other operons and suggests a potential modulatory role in lac operon regulation under specific conditions. However, direct evidence remains limited, necessitating further research.
Influence of Nucleoid-Associated Proteins
NAPs significantly influence lac operon expression by altering DNA supercoiling and bridging DNA regions. For instance:
- H-NS (Histone-like Nucleoid Structuring protein): Typically acts as a global repressor by binding to AT-rich DNA regions and forming nucleoprotein complexes that hinder transcription. While its direct involvement in lac operon repression is not well-documented, its role in global gene silencing suggests a potential indirect influence.
- Fis (Factor for Inversion Stimulation): Functions as an activator or repressor depending on the target gene and growth phase. Fis can modulate DNA topology around the lac operon, thereby affecting RNA polymerase access.
- IHF (Integration Host Factor): Bends DNA upon binding, facilitating or hindering the assembly of transcription complexes.
These proteins dynamically associate with the DNA in response to cellular and environmental cues, influencing the transcriptional landscape, including the lac operon.
Epigenetic Memory and Phenotypic Heterogeneity
One intriguing aspect of bacterial epigenetics is its contribution to epigenetic memory, where cells “remember” previous environmental conditions through heritable modifications. In the context of the lac operon, stochastic variations in regulatory protein concentrations, DNA methylation, and NAP binding patterns can lead to phenotypic heterogeneity in a clonal population.
This bet-hedging strategy enables a subset of cells to maintain lac operon expression even when lactose availability fluctuates, providing a survival advantage in unpredictable environments. Such variability has been demonstrated using single-cell fluorescence microscopy and reporter assays, revealing subpopulations with differing lac operon activities under the same conditions.
Experimental Evidence and Techniques
DNA Methylation Mapping
Techniques such as restriction enzyme digestion with methylation-sensitive enzymes and bisulfite sequencing have been employed to map methylation patterns in bacterial genomes. Advances in nanopore sequencing now allow for real-time detection of base modifications, offering insights into dynamic methylation changes associated with operon regulation.
Chromatin Immunoprecipitation (ChIP)
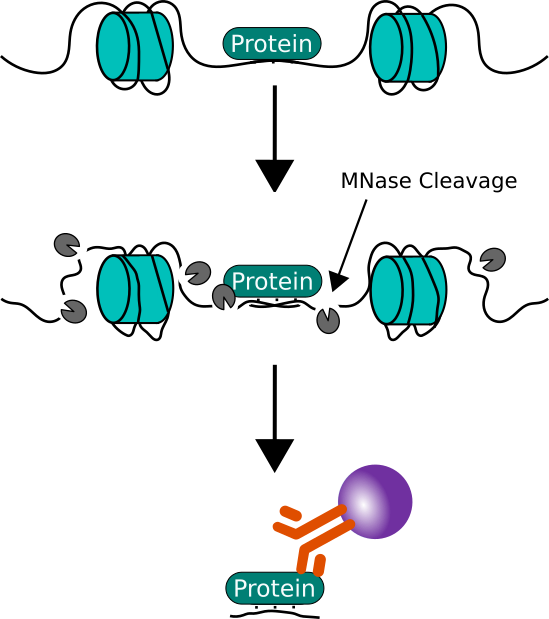
ChIP assays, adapted for prokaryotes, enable the identification of DNA regions bound by NAPs. Coupled with quantitative PCR or sequencing (ChIP-qPCR, ChIP-seq), this technique has elucidated the genome-wide binding patterns of H-NS, Fis, and other NAPs, revealing their involvement in regulating operon expression.
Single-Cell Analysis
Fluorescent reporter constructs and time-lapse microscopy have provided valuable data on lac operon expression variability among individual cells. These studies highlight the role of epigenetic factors in generating phenotypic diversity within bacterial populations.
Future Directions and Applications
Understanding epigenetic regulation in prokaryotes holds promise for various applications:
- Synthetic Biology: Engineering synthetic operons with tunable epigenetic switches for controlled gene expression.
- Antibiotic Resistance: Investigating epigenetic contributions to persistence and resistance phenotypes.
- Biotechnology: Optimizing industrial microbial strains through manipulation of epigenetic regulators.
Further research into the epigenetic modulation of the lac operon will deepen our comprehension of bacterial adaptability and inform the development of novel biotechnological tools.
Conclusion
The regulation of the lac operon in E. coli epitomizes the sophistication of prokaryotic gene control mechanisms. While traditionally attributed to repressor and activator proteins interacting with operator and promoter sequences, emerging evidence suggests that epigenetic-like processes also modulate this system. DNA methylation and nucleoid-associated proteins contribute to the dynamic regulation of gene expression, phenotypic heterogeneity, and environmental adaptability. Although the direct epigenetic regulation of the lac operon remains an evolving field, the integration of classical and modern molecular techniques promises to unravel the complexities of bacterial epigenetics, offering insights with broad implications for microbiology, medicine, and biotechnology.
Frequently Asked Questions (FAQ)
Does DNA methylation directly regulate the lac operon in E. coli?
Not directly. While E. coli uses DNA adenine methylation for regulating other genes, the lac operon itself isn’t directly controlled by methylation. However, nearby methylation sites can indirectly influence regulatory protein binding.
What role do nucleoid-associated proteins (NAPs) play in lac operon regulation?
NAPs like H-NS, Fis, and IHF modulate DNA structure and accessibility, potentially affecting the lac operon’s transcription by altering DNA supercoiling or facilitating/repressing RNA polymerase binding.
Can epigenetic mechanisms cause phenotypic variation in lac operon expression?
Yes. Variations in DNA methylation and NAP binding contribute to phenotypic heterogeneity, allowing some cells in a population to express the lac operon while others do not — a strategy beneficial in fluctuating environments.