Enzymes are the workhorses of the cell, orchestrating the biochemical reactions that sustain life. These biological catalysts speed up reactions by providing an alternative, lower-energy pathway, making life’s processes possible at a reasonable pace. But what happens when these vital enzymes are hampered in their function? Enter the world of enzyme inhibition.
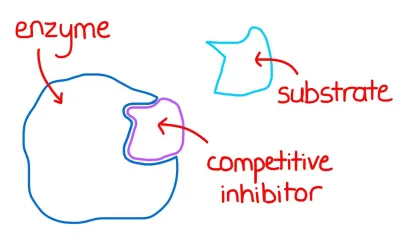
Table of Contents
Enzyme Inhibition: A Molecular Roadblock
Enzyme inhibition occurs when a molecule, called an inhibitor, binds to an enzyme and hinders its ability to catalyze a reaction. Think of it like a traffic jam: the inhibitor is the stalled car, preventing the flow of molecules through the enzymatic “highway”. This blockage can be temporary or permanent, depending on the nature of the inhibitor and its interaction with the enzyme.
Types of Enzyme Inhibitors: A Molecular Diversity
Enzyme inhibitors are classified based on their interaction with the enzyme and their effect on the reaction rate. Here’s a breakdown of the major categories:
Reversible Inhibitors
These inhibitors bind to the enzyme through non-covalent interactions, like hydrogen bonds or electrostatic interactions. This binding is temporary, and the inhibitor can detach, allowing the enzyme to regain its activity.
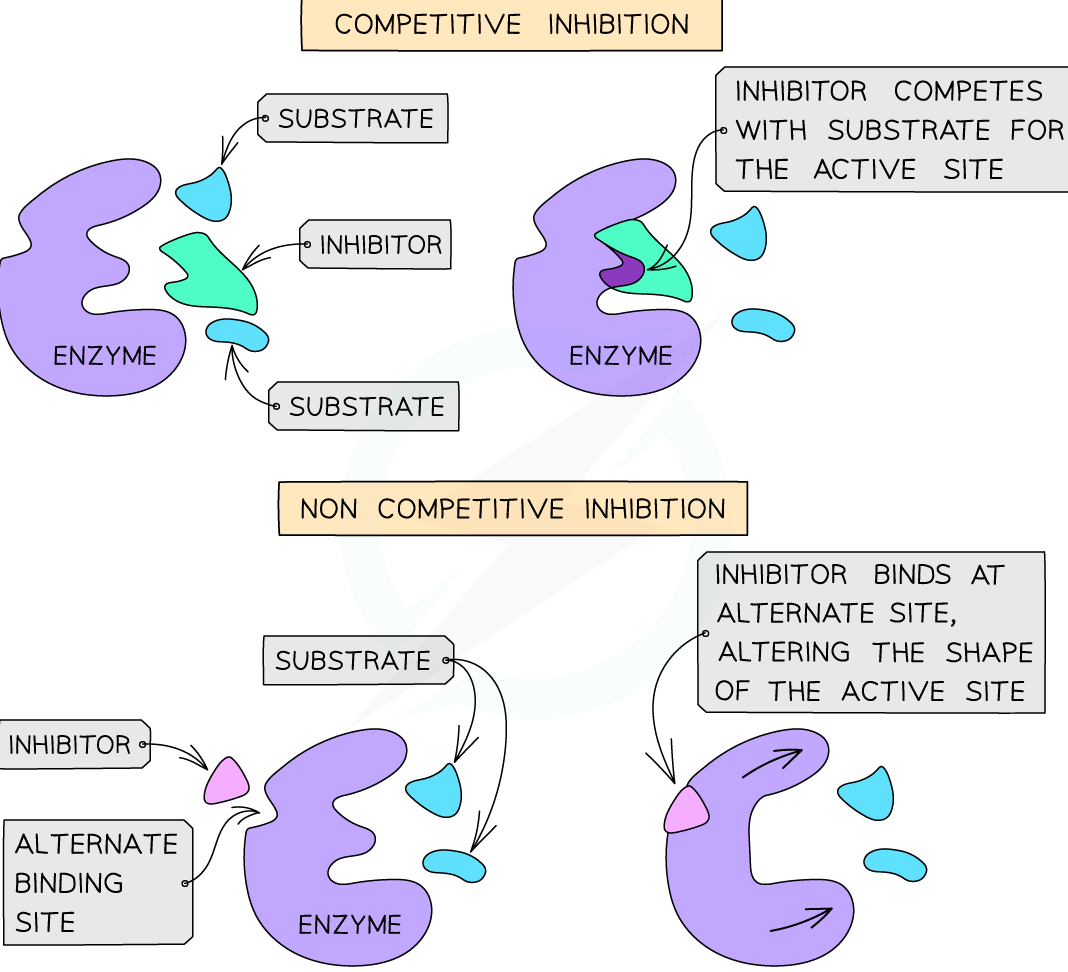
Competitive Inhibition: The inhibitor resembles the enzyme’s natural substrate and competes for binding to the active site. Think of it as a “fake” key trying to fit into the enzyme’s lock. This type of inhibition can be overcome by increasing the concentration of the substrate, essentially outcompeting the inhibitor.
Uncompetitive Inhibition: The inhibitor binds to a site distinct from the active site, but only after the substrate has bound. This binding changes the enzyme’s conformation, rendering it inactive. This type of inhibition is not overcome by increasing substrate concentration.
Noncompetitive Inhibition: The inhibitor binds to a site distinct from the active site, whether or not the substrate is bound. This binding alters the enzyme’s conformation, reducing its catalytic efficiency. This type of inhibition is not overcome by increasing substrate concentration.
Irreversible Inhibitors
These inhibitors bind to the enzyme through covalent bonds, forming a stable complex that permanently disables the enzyme. Think of it as a “superglue” that permanently disables the enzyme’s function.
Mechanism-Based Inhibitors: These inhibitors are initially unreactive but are transformed by the enzyme into a reactive species that covalently modifies the enzyme, leading to irreversible inactivation.
Suicide Inhibitors: A specific type of mechanism-based inhibitor that resembles the substrate and binds to the active site, but then undergoes a reaction catalyzed by the enzyme, generating a reactive species that permanently inactivates the enzyme.
Understanding the Consequences: From Medicine to Metabolism
Enzyme inhibition plays a critical role in biological processes and has far-reaching implications in medicine, agriculture, and industry:
Drug Development: Many drugs function as enzyme inhibitors, targeting specific enzymes involved in disease pathways. For example, aspirin inhibits cyclooxygenase enzymes, reducing inflammation and pain.
Metabolic Regulation: Cells tightly regulate their metabolic processes through enzyme inhibition. For example, feedback inhibition, where the product of a metabolic pathway inhibits an enzyme involved in its own synthesis, helps maintain cellular homeostasis.
Pesticide Action: Many pesticides act as enzyme inhibitors, disrupting essential metabolic processes in pests, leading to their control.
Food Preservation: Enzyme inhibitors are used in food preservation to slow down spoilage reactions. For example, sulfites inhibit enzymes involved in browning reactions in fruits and vegetables.
The Dynamic Dance of Inhibition: From Molecules to Life
The study of enzyme inhibition is an intricate dance between the structure and function of molecules. By understanding the mechanisms of inhibition, we can unravel the secrets of biological processes, develop new therapies, and engineer better enzymes for industrial applications. It’s a testament to the complexity and elegance of life, where even the smallest molecule can have a profound impact on the grand symphony of cellular function.
Frequently Asked Questions(FAQ)
What do you mean by Enzyme inhibition?
Enzyme inhibition is the process by which an inhibitory molecule attaches itself to an enzyme and prevents it from catalyzing a reaction. Imagine the enzymatic “highway” as a traffic congestion, with the inhibitor acting as the halted automobile that is stopping molecules from moving forward. The duration of the blockage can vary based on the inhibitor’s kind and how it interacts with the enzyme.
Write about Reversible Inhibitors?
Reversible Inhibitors are non-covalent interactions such as hydrogen bonds or electrostatic interactions, these inhibitors attach to the enzyme. The inhibitor can separate from the enzyme to restore its function, although this interaction is only transient.
Write about Irreversible Inhibitors?
Irreversible inhibitors attach themselves to the enzyme via covalent bonds to create a stable complex that renders the enzyme inactive for good. Consider it a kind of “superglue” that renders the enzyme inoperable for all time.
Related Questions