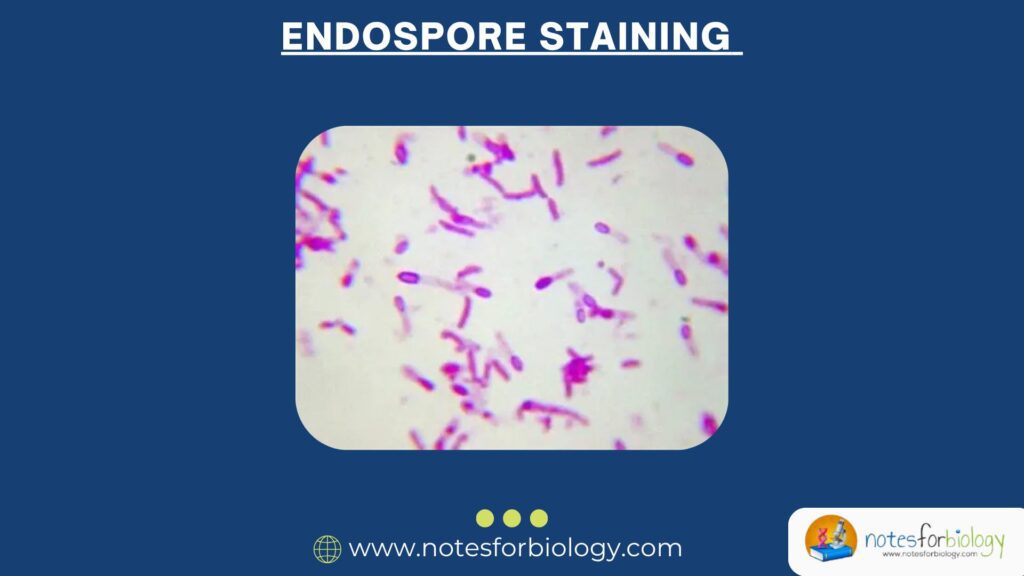
Introduction
Bacteria, like all living organisms, face hostile environments from extreme temperatures to harsh chemicals. To survive such adverse conditions, some bacteria have evolved a powerful survival structure known as an endospore. These structures are tough, dormant, and highly resistant. Detecting them is essential in clinical microbiology, food safety, and industrial hygiene.
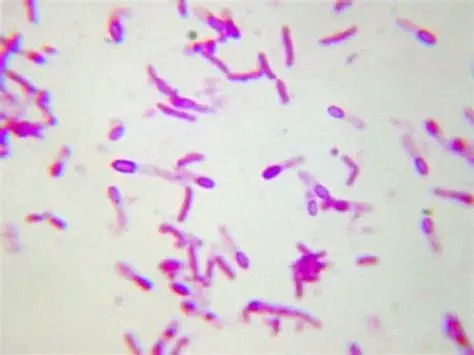
That’s where endospore staining comes in. This powerful technique helps us visualize these otherwise invisible structures under the microscope. In this document, we’ll explore the definition, principles, types of staining, procedures, and interpretation—all in a simple and human-friendly way.
Table of Contents
What Are Endospores?
Endospores are thick-walled, dormant, and non-reproductive structures formed inside certain Gram-positive bacteria like Bacillus and Clostridium species. They are built to withstand:
- High temperatures
- UV radiation
- Desiccation (drying out)
- Chemicals and disinfectants
These protective capsules form when environmental conditions become unfavorable, allowing bacteria to survive until conditions improve.
What is Endospore Staining?
Endospore staining is a microbiological technique used to differentiate endospores from vegetative (active) bacterial cells under a microscope.
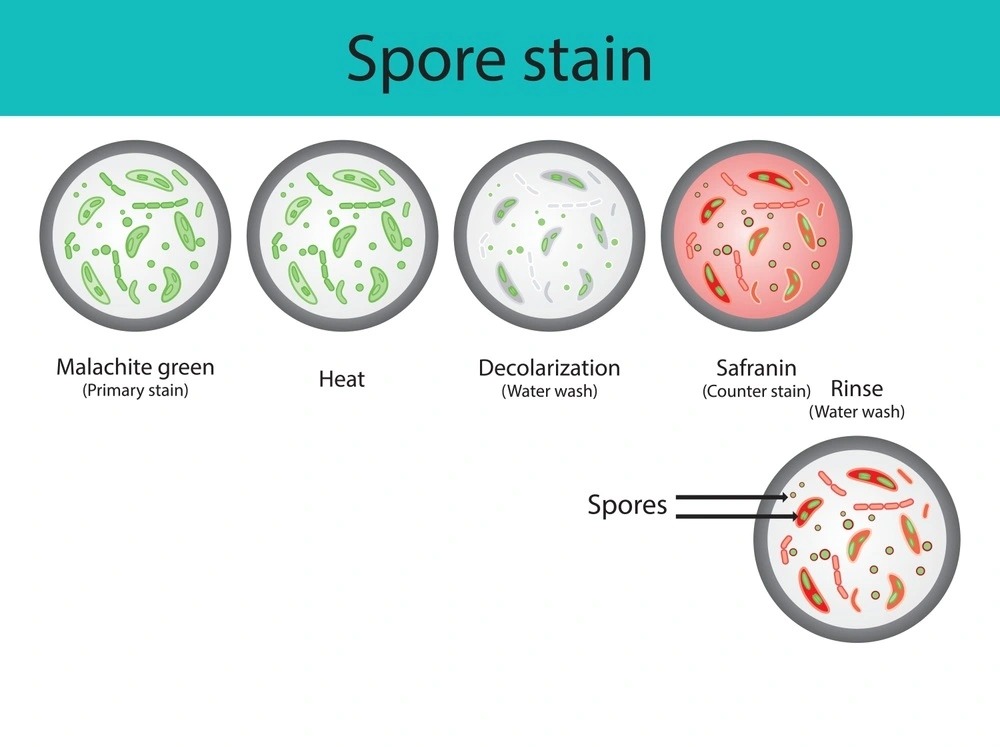
Why stain endospores?
- They are resistant to conventional staining methods.
- Their tough outer covering prevents dye from entering.
- Specific techniques allow us to force stains into the spore structure.
Principle of Endospore Staining
The basic principle behind endospore staining revolves around using heat to drive a primary stain (such as malachite green) into the tough endospore coat.
- Once the dye is forced in, it remains trapped.
- Vegetative cells are then counterstained with a contrasting dye like safranin.
This creates a clear visual contrast:
- Endospores appear green
- Vegetative cells appear pink or red
Types of Endospore Staining
There are several staining techniques, but the two most commonly used are:
1. Schaeffer-Fulton Method (Most popular)
2. Dorner’s Method
Let’s break them down.
1. Schaeffer-Fulton Method
This is the standard technique used in most laboratories.
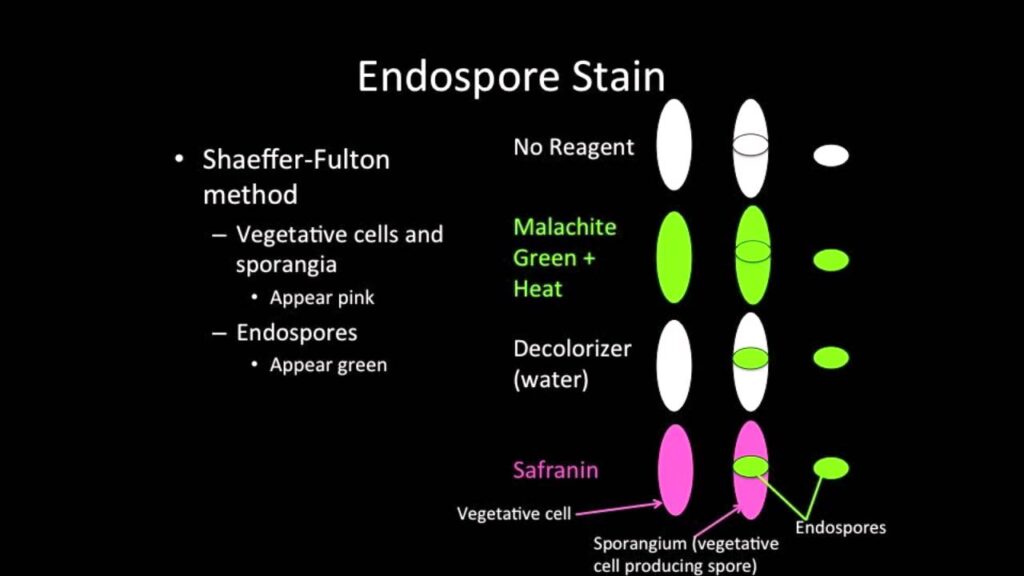
Dyes Used:
- Malachite green (primary stain)
- Safranin (counterstain)
Principle:
Malachite green penetrates the endospore by heat, and safranin stains the surrounding vegetative cells.
2. Dorner’s Method
This is a negative staining technique that uses carbol fuchsin and nigrosin.
Dyes Used:
- Carbol fuchsin (stains the endospore)
- Nigrosin (background stain)
Principle:
Spores are stained red by carbol fuchsin, while the background is stained dark by nigrosin. Vegetative cells appear colorless against the dark background.
Procedure for Schaeffer-Fulton Method
Materials Required:
- Bacterial culture (e.g., Bacillus subtilis)
- Glass slide
- Inoculating loop
- Bunsen burner or hot plate
- Malachite green (5%)
- Safranin (0.5–1%)
- Distilled water
- Microscope
Step-by-Step Procedure:
1. Prepare the Smear
- Place a small drop of water on a clean slide.
- Transfer a small amount of bacterial culture with a sterile loop.
- Spread to form a thin smear.
- Air dry and heat-fix the smear.
2. Apply Malachite Green
- Place the slide on a heat source (e.g., steaming water bath).
- Flood the slide with malachite green.
- Heat the slide gently for 5–10 minutes, keeping it moist (don’t boil or dry out).
3. Cool and Rinse
- Allow the slide to cool.
- Rinse with distilled water to remove excess stain.
4. Counterstain with Safranin
- Apply safranin for 30–60 seconds.
- Rinse with water.
5. Dry and Examine
- Blot dry with bibulous paper.
- View under oil immersion (1000× magnification).
Expected Results:
- Endospores: Green
- Vegetative cells: Red or pink
Procedure for Dorner’s Method
Materials Required:
- Bacterial culture
- Glass slide
- Nigrosin
- Carbol fuchsin
- Bunsen burner
- Water bath
Step-by-Step Procedure:
- Heat a test tube with carbol fuchsin in a boiling water bath.
- Mix a loopful of culture with the stain.
- Allow the mixture to sit for 10 minutes.
- Add a drop of nigrosin on a clean slide.
- Mix the stained culture into the nigrosin.
- Spread the mixture like a thin film.
- Air dry and examine under oil immersion.
Expected Results:
- Endospores: Bright red
- Background: Dark
- Vegetative cells: Colorless or faintly stained
Interpretation of Endospore Staining
When you examine the stained smear under the microscope:
- Green endospores inside pink cells: Active cells producing spores (sporulating cells)
- Free green endospores: Dormant spores released into the environment
- Only pink/red cells: No endospores present (non-sporulating bacteria)
Examples of Endospore-Forming Bacteria
- Bacillus anthracis – causes anthrax
- Clostridium tetani – causes tetanus
- Clostridium botulinum – causes botulism
- Bacillus cereus – food poisoning
- Clostridium difficile – severe diarrhea
These pathogens form spores to persist in hostile environments and resist antibiotics, disinfectants, and heat.
Importance of Endospore Staining
1. Medical Diagnosis
Detects potentially deadly bacteria in clinical samples.
2. Food and Water Safety
Identifies contamination with spore-forming bacteria.
3. Industrial Sterility Testing
Confirms sterilization effectiveness in pharmaceutical and food production.
4. Research and Education
Helps study bacterial survival, resistance, and sporulation.
Limitations of Endospore Staining
- Requires heating, which may damage slides or be dangerous if not done carefully.
- Cannot determine the viability of the endospore (live or dead).
- Some bacteria may produce subterminal or terminal spores, which require experience to recognize.
Tips for Better Staining
- Keep the smear thin—thicker smears trap dye unevenly.
- Don’t overheat the slide, as it may cause artifacts.
- Use fresh cultures for better visualization.
- Always observe under oil immersion for clarity.
Comparison: Schaeffer-Fulton vs. Dorner’s Method
| Feature | Schaeffer-Fulton Method | Dorner’s Method |
|---|---|---|
| Primary Stain | Malachite green | Carbol fuchsin |
| Counterstain | Safranin | Nigrosin (background) |
| Heat Required | Yes | Yes |
| Appearance of Spores | Green | Bright red |
| Background | Clear or pink cells | Dark |
| Ease of Use | Widely used in labs | Less common |
Conclusion
Endospore staining is a vital microbiological technique that reveals the presence of one of the most resilient forms of life. By using specific dyes and heating, scientists and healthcare professionals can differentiate tough, dormant spores from active bacterial cells. This knowledge is essential for diagnosing infections, ensuring food safety, validating sterilization processes, and understanding bacterial survival.
With either the classic Schaeffer-Fulton method or the more artistic Dorner’s method, endospore staining continues to play a foundational role in microbiology education and practice.
Three Key Summary
- Endospores are heat-resistant, dormant bacterial structures, and require specialized staining to visualize.
- The Schaeffer-Fulton method uses malachite green and safranin to differentiate spores and vegetative cells.
- This staining is critical for medical, environmental, and industrial microbiology.
Frequently Asked Questions (FAQs)
What is the purpose of endospore staining?
To detect and distinguish endospores from vegetative bacterial cells under a microscope.
Why don’t endospores stain easily?
They have a tough, keratin-like coat that resists regular staining methods.
Which bacteria form endospores?
Bacillus and Clostridium species are the most well-known spore-formers.
Related Articles