Introduction
Microorganisms play a vital role in many biotechnological processes. One of the key methods for cultivating these organisms, especially for industrial and research purposes, is the continuous culture system. Unlike batch culture, where microorganisms are grown in a fixed volume of nutrients, continuous culture provides a constant environment where fresh nutrients are added and waste is removed consistently. This method allows for the maintenance of cells in a steady-state condition for extended periods.

In this article, we will explore the concept of continuous culture in detail, covering its definition, core principles, operational processes, types, real-life applications, and some of its limitations. We will also examine how continuous culture contributes to modern science, medicine, and industry.
Table of Contents
Definition
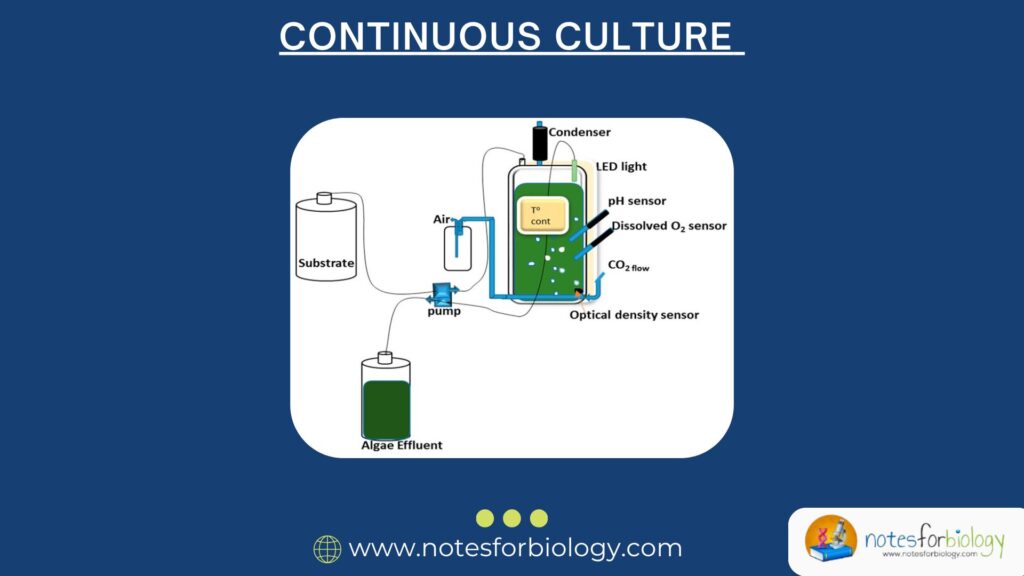
Continuous culture is a cultivation technique in which microorganisms are grown in a controlled system with a continuous supply of fresh nutrient medium and simultaneous removal of spent medium and microbial biomass.
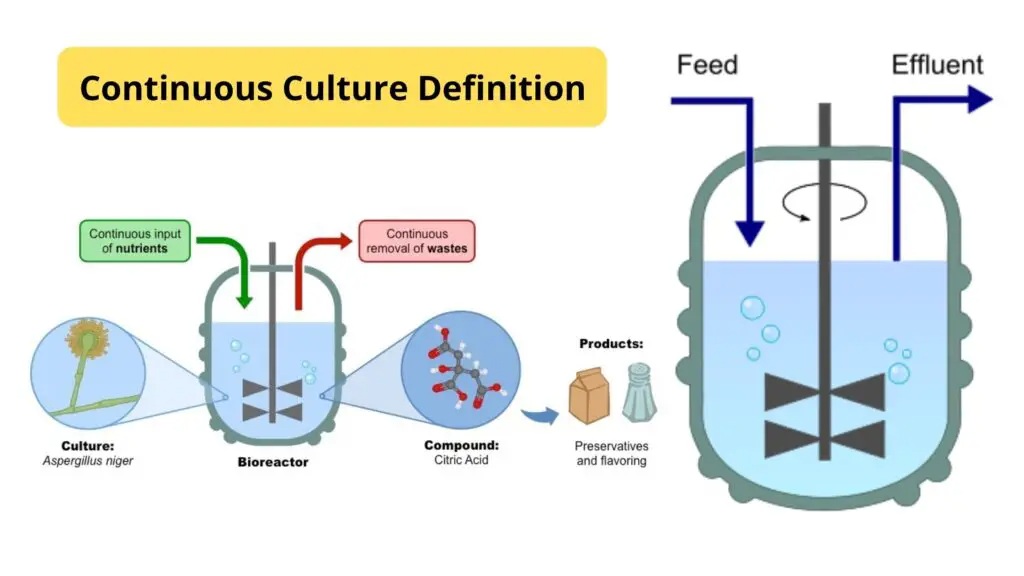
Key Features:
- Steady-state condition: Nutrients and environmental conditions are kept constant.
- Open system: Continuous input and output.
- Long-term cultivation: Ideal for physiological and metabolic studies.
Principle of Continuous Culture
The underlying principle of continuous culture is based on the concept of growth rate control by limiting one of the essential nutrients.
Core Concepts:
- Limiting Nutrient: Only one essential nutrient is provided in limiting quantity to control growth rate.
- Dilution Rate (D): The rate at which fresh medium is added and culture is removed. It influences the microbial growth.
- Steady-State: Achieved when the rate of microbial growth equals the dilution rate, leading to a constant biomass concentration.
The Monod equation often describes the relationship between specific growth rate (μ) and limiting substrate concentration (S):
μ = (μmax × S) / (Ks + S)
Where:
- μ = Specific growth rate
- μmax = Maximum specific growth rate
- Ks = Half-saturation constant
- S = Substrate concentration
Process of Continuous Culture
Continuous culture systems require careful control and monitoring to maintain a stable environment.
Steps Involved:
1. Sterilization
The fermenter, medium, and any instruments are sterilized to avoid contamination.
2. Inoculation
A known quantity of microbial culture is added to the sterilized medium.
3. Medium Supply
Fresh nutrient medium is added continuously at a controlled rate.
4. Culture Removal
An equal amount of culture (medium + cells) is removed to maintain constant volume.
5. Monitoring and Control
Parameters like pH, temperature, dissolved oxygen, and cell density are monitored.
Types of Continuous Culture
There are several variations of continuous culture systems, each with unique advantages and suited for different research and industrial needs.
1. Chemostat
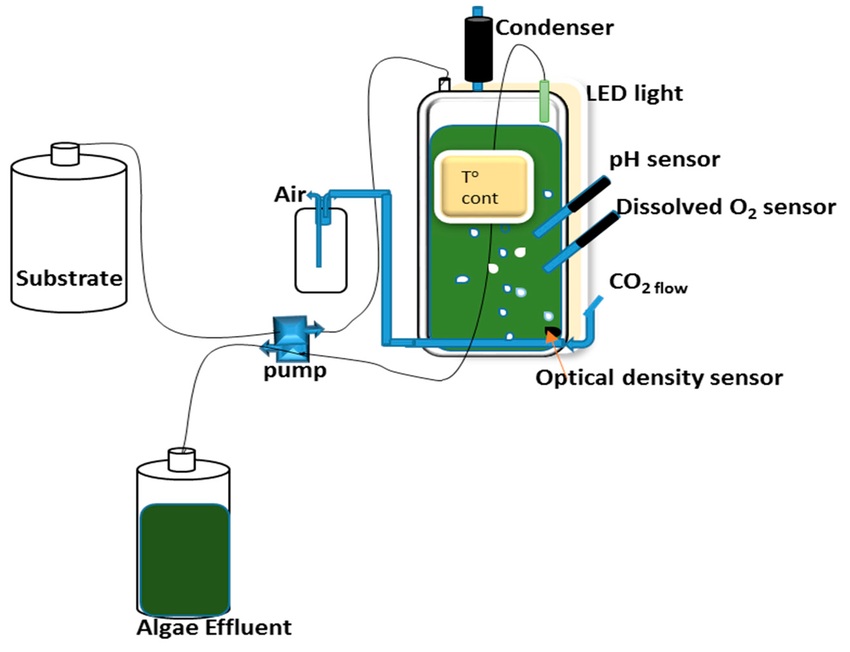
- Most common type.
- Growth rate is controlled by the limiting nutrient.
- Operated at a fixed dilution rate.
Applications:
- Study of microbial physiology and kinetics.
- Evolutionary and ecological studies.
2. Turbidostat
- Growth is maintained at a constant turbidity (cell density).
- Dilution rate varies automatically.
Applications:
- Fast-growing microorganisms.
- Useful for studying cells under near-maximal growth.
3. Auxostat
Regulated by a feedback loop depending on a specific parameter (e.g., pH, oxygen).
Applications:
Fermentations requiring variable control conditions.
4. Fed-Batch Culture (Semi-continuous)
- A compromise between batch and continuous culture.
- Fresh medium is added without removing culture.
Applications:
- Industrial production of antibiotics and enzymes.
Applications of Continuous Culture
It has extensive applications in both basic research and industrial biotechnology.
1. Industrial Fermentation
- Used for large-scale production of products like alcohol, antibiotics, amino acids, enzymes, and organic acids.
- Continuous operation increases productivity and consistency.
2. Pharmaceutical Industry
- Production of vaccines and therapeutic proteins under controlled conditions.
- Long-term culture stability ensures consistent product quality.
3. Bioremediation Studies
Growth of pollutant-degrading bacteria in continuous systems to study their capabilities.
4. Microbial Physiology Research
Study of how microbes respond to changes in nutrients, oxygen, or temperature over time.
5. Environmental Microbiology
Modeling ecosystems, such as wastewater treatment systems, where continuous microbial growth is essential.
6. Biofuel Production
Cultivation of algae or bacteria in continuous systems to produce ethanol, methane, or hydrogen efficiently.
7. Synthetic Biology and Genetic Engineering
Continuous expression of genetically engineered pathways in bacteria or yeast.
Limitations of Continuous Culture
Despite its many advantages, continuous culture has several limitations:
1. Contamination Risk
Since the system is open and operates for long periods, contamination is a major issue.
2. Genetic Instability
Long-term cultivation may lead to mutations or selection of non-productive variants.
3. Technical Complexity
Requires sophisticated equipment and monitoring systems.
4. Foaming and Biofilm Formation
- Foaming can interfere with measurements.
- Biofilms may form on bioreactor surfaces, affecting culture conditions.
5. Cost and Maintenance
Initial setup and continuous operation costs are higher compared to batch systems.
6. Not Ideal for All Microbes
Some microorganisms may not grow well under continuous conditions or may require specific environmental triggers.
Comparison: Continuous Culture vs. Batch Culture
| Feature | Continuous Culture | Batch Culture |
|---|---|---|
| System Type | Open | Closed |
| Nutrient Supply | Constant | Fixed |
| Waste Removal | Continuous | None |
| Culture Duration | Long-term | Short-term |
| Growth Phase Control | Steady-state | All phases (lag to death) |
| Risk of Contamination | Higher | Lower |
| Equipment | Complex | Simple |
Conclusion
Continuous culture has revolutionized how we study microorganisms and produce biological products. It offers a powerful tool for maintaining steady-state microbial growth, which is crucial for reproducibility and industrial efficiency. From pharmaceutical production to environmental applications, it has proven to be a versatile method.
However, it is not without its challenges. Technical complexity, contamination risks, and cost are major concerns. Despite these, the benefits far outweigh the limitations when managed properly.
As technology advances, this systems will become more automated and accessible, opening new frontiers in biotechnology, medicine, and environmental science.
FREQUENTLY ASKED QUESTIONS
What is a chemostat?
A chemostat is a type of continuous culture system where the growth rate of microorganisms is controlled by limiting the concentration of one essential nutrient.
Why is continuous culture preferred in industry?
It provides consistent product yield, long-term cultivation, and efficient use of resources.
Can all microbes be grown in continuous culture?
No. Some microbes require complex or changing environments and are better suited to batch culture.
Related Articles