Serological tests remain one of the most important tools in immunology for detecting the presence of antibodies or antigens within a patient’s serum. These diagnostic tests help confirm exposure to infectious agents, monitor disease progression, and evaluate immune responses. Among the various serological techniques available, the complement fixation test (CFT) holds historical and clinical significance.
CFT was one of the earliest methods developed for detecting antigen-antibody reactions via the use of complement proteins. Though modern methods have emerged, complement fixation remains a valuable reference technique, especially in specific epidemiological and research contexts.
Summary of Complement Fixation Test
- Complement Fixation Test (CFT) is a serological assay that detects specific antibodies or antigens by observing whether complement proteins are fixed by immune complexes in the presence of known antigens or antibodies.
- A positive result is indicated by the absence of hemolysis in an indicator system of sensitized sheep red blood cells, showing that complement was consumed by antigen-antibody complexes.
- Though largely replaced by modern tests like ELISA and PCR, CFT remains valuable for certain infectious disease diagnoses, autoimmune assessments, and historical epidemiological studies.
Table of Contents
Overview of Serological Testing in Immunology
Serological tests detect the presence of antibodies or antigens in biological fluids, typically serum. These assays help determine whether an individual has been exposed to a pathogen, produced specific antibodies, or currently harbors circulating antigens. Immunologists employ various serological methods, including agglutination tests, ELISA, immunofluorescence, and complement fixation.
By evaluating humoral immune responses, these tests assist in diagnosing infections, autoimmune diseases, and immune deficiencies. Their sensitivity, specificity, and adaptability have made them indispensable in both clinical and research laboratories.
Historical Background and Significance of Complement Fixation Test (CFT)
The complement fixation test was introduced in 1909 by Jules Bordet and Octave Gengou while studying the immune response to bacteria. This test became a cornerstone in infectious disease serology for several decades. It was widely used to diagnose diseases such as syphilis (via the Wassermann test) and viral infections long before the advent of enzyme-based immunoassays.
Though partially replaced by modern methods, CFT retains historical importance as the first test to exploit the complement system’s role in immunity for laboratory diagnostics. It established foundational principles for serological testing still applied today.
What is a Complement Fixation Test?
To fully understand CFT, it’s important to grasp its definition, the role of complement in immunity, and its diagnostic applications.
Definition and Basic Concept
A complement fixation test is a serological assay that detects the presence of specific antibodies or antigens in a patient’s serum by assessing their ability to fix complement proteins in the presence of their corresponding antigen or antibody. If complement is consumed (fixed) by the immune complexes formed, it indicates the presence of the target antibody or antigen.
The assay relies on a two-stage reaction where complement fixation occurs in the first stage, and an indicator system, typically sheep red blood cells (SRBCs), reveals whether complement remains in the second.
The Role of Complement in Immunity
Complement is a group of serum proteins that enhances immune responses by promoting inflammation, opsonizing pathogens, and lysing foreign cells. When antibodies bind to antigens, complement proteins attach to the immune complexes and become activated, leading to a cascade of reactions that can culminate in cell lysis.
This ability of complement to participate in antigen-antibody reactions makes it a useful tool in laboratory diagnostics for identifying specific immune responses.
Importance of Complement Fixation in Diagnosis
Complement fixation provides indirect evidence of infection by detecting circulating antibodies against specific pathogens. It is particularly useful for infections where direct detection of the microorganism is difficult or where antibody levels provide better insight into disease history or progression.
Its ability to detect both recent and past infections, combined with high specificity, has made it an important diagnostic tool, especially for viral diseases, parasitic infections, and some bacterial conditions.
Principle of the Complement Fixation Test
The complement fixation test operates on immunological principles involving antigen-antibody interactions and complement activation. Understanding these principles is key to interpreting test results.
Overview of Complement Activation Pathways
Complement can be activated via three pathways: the classical, alternative, and lectin pathways. The classical pathway is initiated when antibodies, primarily IgG or IgM, bind to antigens, creating immune complexes that recruit and activate complement proteins.
CFT specifically uses the classical pathway, as it relies on antibody-mediated complement activation to determine whether specific antibodies or antigens are present in a test sample.
How Antigen-Antibody Reactions Utilize Complement
In CFT, when a patient’s serum containing specific antibodies is mixed with its corresponding antigen, immune complexes form. Complement proteins bind to these complexes and become activated, eventually being consumed (fixed) in the reaction.
If complement remains unbound, it will react with an added indicator system, leading to hemolysis, which helps identify whether complement was fixed in the first reaction stage.
Basis for Detecting Specific Antibodies or Antigens
The presence or absence of hemolysis in the indicator system serves as the readout for CFT. If hemolysis occurs, it means complement was not fixed in the first stage and remained free to lyse indicator red blood cells in the second stage. No hemolysis indicates complement was fixed by antigen-antibody complexes, confirming the presence of the target antibody or antigen in the sample.
Procedure of the Complement Fixation Test
The CFT involves carefully controlled laboratory procedures, requiring precise reagents, sample preparation, and stepwise execution to ensure accurate results.
Required Reagents and Materials
Essential components for CFT include complement (often guinea pig serum), specific antigens, patient serum, sheep red blood cells (SRBCs), hemolysin (anti-SRBC antibody), and buffered saline. Fresh reagents, properly calibrated pipettes, and appropriate incubation conditions are critical for reliable assay performance.
Preparation of Serum Samples
Patient serum samples are collected, typically by venipuncture, and separated by centrifugation. Clear, hemolysis-free serum is essential to prevent interference in the complement fixation reaction.
Heat Inactivation of Complement in Serum
Naturally occurring complement in patient serum must be inactivated by heating at 56°C for 30 minutes. This ensures that only the added exogenous complement participates in the assay, eliminating variability due to endogenous complement levels.
Sensitization of Sheep Red Blood Cells (SRBCs)
Sheep red blood cells are mixed with a hemolysin (antibody against SRBCs) to form sensitized cells. These cells serve as an indicator system, revealing whether complement was fixed in the earlier stage of the test.
Step-by-Step Test Protocol
The test proceeds in two main stages:
First Stage: Complement Fixation
In the first stage, patient serum, specific antigen, and exogenous complement are mixed and incubated. If antibodies are present, antigen-antibody complexes form and fix the complement.
Second Stage: Indicator System Reaction
Sensitized SRBCs are then added. If complement was fixed in the first stage, no hemolysis occurs. If complement remains free, it lyses the SRBCs, producing visible hemolysis that indicates a negative test result.
Types of Complement Fixation Tests
Different variations of CFT have been developed to suit various diagnostic needs, ranging from standard qualitative detection to more refined quantitative assays.
Direct Complement Fixation Test
In this version, complement fixation occurs directly in the presence of antigen, patient serum, and complement. The outcome is read using the indicator system, and hemolysis patterns determine test results.
Indirect (Indirect Hemolysis) Complement Fixation Test
Here, a two-step system is used where antigen-antibody complexes are first formed, followed by the addition of complement and the indicator system. This variation improves sensitivity and specificity for certain infections.
Modified or Quantitative Complement Fixation Test
This type uses graded dilutions of patient serum to quantify antibody titers. The highest dilution showing no hemolysis is recorded as the complement-fixing antibody titer, providing semi-quantitative diagnostic information.
Interpretation of Complement Fixation Test Results
CFT results depend on the degree of hemolysis observed in the indicator system and must be interpreted carefully in the clinical context.
Positive vs Negative Test Outcomes
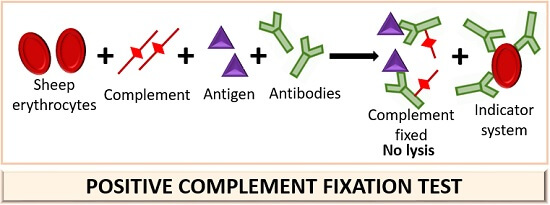
A positive CFT is indicated by the absence of hemolysis, meaning complement was fixed by antigen-antibody complexes. A negative result involves visible hemolysis, signifying no specific antibodies were present to fix complement in the first reaction stage.
Grading the Degree of Hemolysis
Hemolysis can be graded on a scale from 0% (complete complement fixation) to 100% (complete hemolysis), allowing for semi-quantitative assessment of antibody levels. Intermediate levels may suggest weak or borderline-positive reactions.
Factors Influencing Test Accuracy
Test accuracy can be affected by improper serum inactivation, degraded complement, incorrect antigen concentrations, or technical errors in SRBC sensitization. Strict quality control is vital to minimize false positives or negatives.
Applications of Complement Fixation Test
Complement fixation tests have been widely applied in clinical and research laboratories due to their ability to detect both antibodies and antigens in serum. Though gradually replaced by newer techniques, CFT remains relevant for certain pathogens and epidemiological studies where rapid, cost-effective serodiagnosis is needed.
Its flexibility allows it to be adapted for diverse clinical applications, including infectious disease diagnosis, autoimmune condition assessment, and therapeutic monitoring.
Diagnosis of Infectious Diseases (e.g., Syphilis, Viral Infections)
Historically, one of the primary applications of CFT was in the detection of infectious diseases. The Wassermann test for syphilis is a classic example, which utilized CFT principles for serodiagnosis. It has also been employed in detecting antibodies against viruses such as influenza, mumps, measles, coxsackievirus, and herpesviruses.
In regions where advanced diagnostic methods are less accessible, CFT remains a useful tool for confirming exposure to these infectious agents, particularly for diseases that elicit strong, complement-fixing antibody responses.
Autoimmune Disease Detection
CFT can also assist in identifying autoimmune diseases where autoantibodies fix complement upon interacting with self-antigens. For example, in conditions like systemic lupus erythematosus (SLE), complement-fixing immune complexes may be present in circulation and can be detected through CFT-based techniques.
This application provides insights into disease activity and immune complex-mediated tissue injury, complementing other immunological assays.
Monitoring Disease Progression and Treatment Response
Because CFT can detect both active and past infections based on the presence and titers of complement-fixing antibodies, it serves as a valuable tool for monitoring disease progression. Serial measurements of antibody titers via quantitative CFT can reveal changes in immune status over time, reflecting either improvement or worsening of a condition.
It has been used in therapeutic follow-ups for certain viral, parasitic, and fungal infections, as well as in post-vaccination antibody monitoring in experimental settings.
Advantages of Complement Fixation Test
Despite the advent of newer immunoassays, CFT retains several advantages that make it suitable for specific applications and laboratory settings.
High Specificity and Sensitivity
CFT is highly specific due to its dependence on precise antigen-antibody interactions. The involvement of complement, which only binds to immune complexes, adds an additional layer of specificity, reducing non-specific reactions.
When performed under optimal conditions, CFT is sensitive enough to detect low levels of circulating antibodies, making it useful for early or latent infection detection.
Versatility in Detecting Both Antibodies and Antigens
Unlike some assays limited to detecting either antibodies or antigens, CFT can be adapted to identify either, depending on how the test is configured. By adjusting reagent combinations, it is possible to confirm the presence of circulating antigens in addition to antibodies, which enhances its diagnostic versatility.
This dual-function capability has historically made CFT a valuable method in broad-based infectious disease screening programs.
Cost-Effective for Large-Scale Screening
CFT does not require sophisticated equipment, expensive enzyme conjugates, or radioactive reagents, making it a relatively affordable option for large-scale screening, particularly in resource-limited settings. The reagents are readily available, and with proper technique, multiple samples can be tested simultaneously, improving laboratory efficiency.
Limitations and Challenges of Complement Fixation Test
Despite its merits, the complement fixation test presents several limitations that restrict its routine use in modern diagnostics.
Requirement of Fresh Complement
A major drawback of CFT is its reliance on fresh complement, typically derived from guinea pig serum, which is both perishable and biologically variable. Complement activity can deteriorate over time or with improper storage, leading to inconsistent results.
Maintaining a reliable source of active complement requires careful handling, frequent potency testing, and strict storage conditions.
Potential for False Positives/Negatives
CFT outcomes can be influenced by multiple factors, including improper serum inactivation, contamination, suboptimal antigen preparation, or degraded complement. Hemolysis due to factors unrelated to immune complex formation, such as technical error or hemolysin instability, can lead to false-positive results.
Conversely, weak or degraded complement or insufficient antibody levels might result in false negatives. Therefore, rigorous control testing is essential for accurate interpretation.
Labor-Intensive Procedure
Compared to modern ELISA or PCR-based assays, CFT is time-consuming and technically demanding. It requires multiple preparation steps including serum inactivation, SRBC sensitization, complement handling, and titration each prone to technical variation.
This labor-intensive nature, combined with the need for experienced personnel, limits its widespread use in high-throughput diagnostic laboratories today.
Modern Alternatives to Complement Fixation Test
Advances in immunodiagnostics have led to the development of alternative techniques offering greater sensitivity, specificity, speed, and automation than CFT. These modern assays have largely replaced CFT in most clinical laboratories.
ELISA (Enzyme-Linked Immunosorbent Assay)
ELISA is now the preferred serological method in most settings. It detects antigens or antibodies using enzyme-labeled reagents and produces a colorimetric, fluorescent, or luminescent readout.
ELISA is more sensitive, scalable, and compatible with automation than CFT and does not require fresh complement or labor-intensive protocols. It’s widely used for diagnosing infectious, autoimmune, and allergic conditions.
Radioimmunoassay (RIA)
RIA employs radiolabeled antigens or antibodies to detect immune complexes. It offers high sensitivity and precision but involves radioactive materials, limiting its use to specialized research laboratories with appropriate safety infrastructure.
Despite its declining use, RIA remains valuable for certain endocrine and immunological investigations where exceptional assay sensitivity is required.
PCR-Based Diagnostic Methods
Polymerase chain reaction (PCR) has revolutionized infectious disease diagnostics by detecting pathogen DNA or RNA directly, bypassing the need for immune response detection. PCR is highly specific and can detect pathogens at very low concentrations.
Its rapid turnaround and ability to identify acute infections before antibody production make it a superior option for many clinical applications previously handled by CFT.
Safety and Ethical Considerations
As with all immunodiagnostic techniques, ethical and biosafety guidelines must be followed during CFT procedures to ensure the safety of personnel and adherence to humane research practices.
Use of Animal-Derived Components (SRBCs)
CFT relies on animal-derived reagents such as sheep red blood cells and guinea pig complement. The procurement and use of these materials must comply with ethical standards for animal welfare.
Laboratories are encouraged to source reagents from certified, ethically-managed suppliers and follow institutional guidelines regarding animal component handling and disposal.
Handling Human Serum Samples
Human biological samples pose potential biohazard risks. Laboratory staff must use appropriate personal protective equipment (PPE) and follow biosafety guidelines for specimen handling, storage, and disposal.
Proper sample anonymization and patient consent are also required for research applications to maintain ethical standards in diagnostic practice.
Recent Developments in Complement-Based Assays
Recent years have seen the emergence of refined complement activity tests and complement-targeted therapeutics, expanding the clinical utility of complement studies beyond traditional fixation tests.
Automated Complement Activity Testing
Automated systems for measuring complement activity, such as CH50 and AH50 assays, have replaced manual CFT in many diagnostic laboratories. These assays provide standardized, reproducible measurements of complement function in patient serum.
Such automation enhances assay consistency, improves safety by minimizing manual handling of biohazards, and delivers faster results.
Complement-Targeted Therapeutics
Therapies targeting the complement cascade are now under development for treating autoimmune and inflammatory conditions. Agents like eculizumab, a monoclonal antibody against complement component C5, have shown success in managing diseases such as paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS).
This growing interest in complement modulation has renewed research into complement assays, including more sophisticated versions of fixation-based tests for monitoring therapy efficacy.
Conclusion
The complement fixation test represents a landmark achievement in serological testing, establishing foundational principles that paved the way for modern immunodiagnostic techniques. Although labor-intensive and dependent on fresh biological reagents, its high specificity and dual capacity for detecting both antibodies and antigens gave it a lasting role in infectious disease and autoimmune diagnostics.
Today, advanced methods like ELISA, PCR, and automated complement assays have largely supplanted CFT in routine practice. Nonetheless, understanding the principles, strengths, and limitations of CFT remains essential, as it continues to serve in specific applications and as a historical reference in the development of immunological diagnostics.
Frequently Asked Questions (FAQ)
What diseases can be diagnosed using a complement fixation test?
CFT can diagnose infections like syphilis, Q fever, measles, histoplasmosis, and some viral and autoimmune diseases.
Why is heat inactivation of serum necessary in CFT?
It removes natural complement in serum to prevent interference, ensuring only added complement participates in the test.
What factors can affect complement activity in tests?
Complement activity is influenced by storage conditions, temperature changes, contamination, reagent age, and pH variations.
Related Contents
Competitive ELISA: Principle, Protocol, Applications, and Animation
Communicable Vs Non-communicable Diseases- Definition, 17 Differences, Examples