Chemiosmosis is a fundamental biological process that plays a key role in energy production in living cells. It is the movement of ions (usually hydrogen ions, H⁺) across a selectively permeable membrane, down their electrochemical gradient. This process is central to the production of adenosine triphosphate (ATP), the energy currency of the cell. Chemiosmosis occurs during cellular respiration and photosynthesis and is critical to life itself.
What is Chemiosmosis?
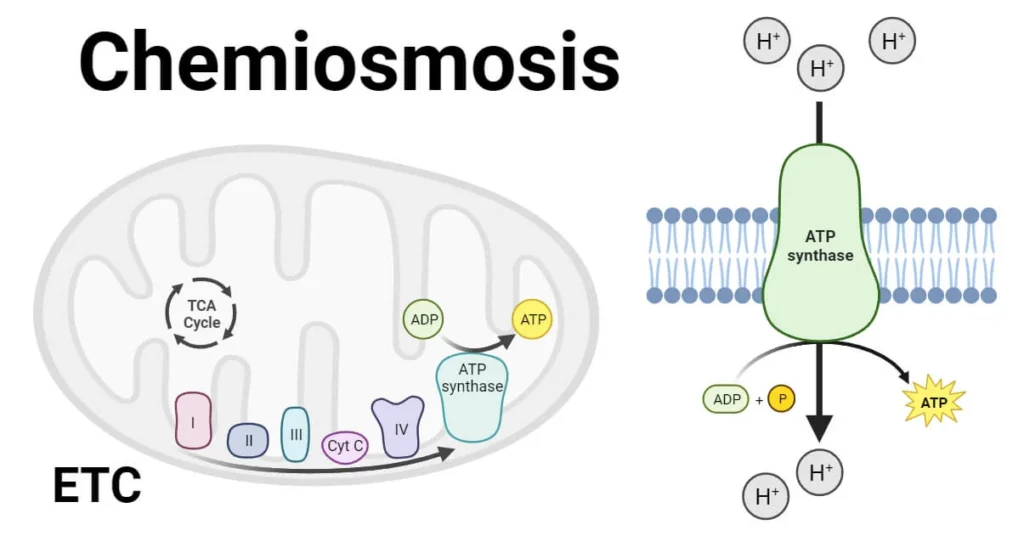
Chemiosmosis refers to the movement of ions (specifically protons) across a membrane through a protein channel, which is used to drive the synthesis of ATP. This process is powered by a proton gradient created by the electron transport chain. The energy released as electrons pass through the chain is used to pump protons across the membrane, creating an electrochemical gradient. As protons flow back through ATP synthase, the energy is captured to convert ADP and inorganic phosphate (Pi) into ATP.
Summary of Chemiosmosis
- The centrosome is a key organelle in animal cells that organizes microtubules and helps during cell division.
- It is made of two centrioles and surrounding protein material that guide the formation of the cell’s internal structure.
- The centrosome ensures proper chromosome separation, cell shape, and the formation of cilia, and its malfunction can lead to diseases like cancer and developmental disorders.
Table of Contents
Historical Background of Chemiosmosis

The chemiosmotic theory was proposed by Peter Mitchell in 1961. At the time, the mechanism of ATP production was not fully understood. Mitchell’s theory explained how the electron transport chain and proton gradient are used to drive ATP synthesis. Despite initial skepticism, his theory was later validated and earned him the Nobel Prize in Chemistry in 1978.
Components Involved in Chemiosmosis
- Electron Transport Chain (ETC): A series of protein complexes located in the inner mitochondrial membrane (in eukaryotes) or plasma membrane (in prokaryotes) that pass electrons and pump protons.
- Proton Gradient: Created by the ETC, this gradient represents stored potential energy.
- ATP Synthase: An enzyme embedded in the membrane that uses the proton motive force to synthesize ATP.
- Membranes: The process occurs across the inner mitochondrial membrane (in cellular respiration) and thylakoid membrane (in photosynthesis).
Chemiosmosis in Cellular Respiration
Cellular respiration includes glycolysis, the Krebs cycle, and oxidative phosphorylation. Chemiosmosis is a part of oxidative phosphorylation:
- Location: Inner mitochondrial membrane.
- Process:
- NADH and FADH₂ donate electrons to the ETC.
- Electrons travel through complexes I-IV, releasing energy.
- This energy pumps protons from the mitochondrial matrix into the intermembrane space.
- A high concentration of protons builds up, forming a proton motive force.
- Protons flow back into the matrix through ATP synthase.
- The energy from this flow is used to convert ADP + Pi → ATP.
- Final Electron Acceptor: Oxygen accepts electrons and combines with protons to form water.
Chemiosmosis in Photosynthesis
Photosynthesis occurs in the chloroplasts of plant cells. Light reactions drive chemiosmosis:
- Location: Thylakoid membrane.
- Process:
- Light excites electrons in chlorophyll.
- Electrons travel through the photosynthetic electron transport chain.
- Energy is used to pump protons from the stroma into the thylakoid lumen.
- Proton gradient builds inside the thylakoid lumen.
- Protons flow back into the stroma via ATP synthase.
- ATP is produced for use in the Calvin cycle.
Role of Proton Motive Force (PMF)
Proton motive force is the combination of:
- Electrical potential (voltage across the membrane)
- Chemical gradient (difference in proton concentration)
PMF drives the rotation of ATP synthase and thereby enables ATP synthesis. It is also used for other cellular processes such as active transport and flagellar rotation in bacteria. This electrochemical force acts like a biological battery, storing energy used for multiple cellular activities beyond just ATP generation.
ATP Synthase: The Molecular Motor
ATP synthase is a complex protein with two main parts:
- F₀ Portion: Embedded in the membrane; forms a channel for proton flow.
- F₁ Portion: Protrudes into the mitochondrial matrix or stroma; synthesizes ATP.
As protons flow through F₀, it causes rotation that mechanically drives the F₁ portion to bind ADP and Pi, forming ATP. This rotary mechanism is one of the most fascinating examples of molecular machinery in biology.
Efficiency of Chemiosmosis
Chemiosmosis is a highly efficient process:
- Approximately 34 of the 38 ATP molecules produced during aerobic respiration come from chemiosmosis.
- In photosynthesis, chemiosmosis provides the ATP needed to convert carbon dioxide into glucose.
Its efficiency is a testament to how evolution has optimized energy conservation in biological systems.
Comparison: Cellular Respiration vs Photosynthesis
| Feature | Cellular Respiration | Photosynthesis |
|---|---|---|
| Location | Mitochondrial membrane | Thylakoid membrane |
| Electron Source | NADH, FADH₂ | Water |
| Final Electron Acceptor | Oxygen | NADP⁺ |
| ATP Use | Used by cell | Used in Calvin cycle |
This comparison highlights how nature uses a similar mechanism in two different energy pathways—one that breaks down food and one that builds it.
Applications of Chemiosmosis
- Medicine: Understanding mitochondrial diseases linked to defects in the ETC.
- Biotechnology: Targeting ATP production pathways in drug development.
- Agriculture: Enhancing photosynthesis efficiency to improve crop yield.
- Bioenergetics: Studying energy flow and storage in biological systems.
Disorders Related to Chemiosmosis
- Leigh Syndrome: A genetic disorder affecting mitochondrial function.
- Mitochondrial Myopathies: Muscle diseases caused by defective mitochondria.
- Oxidative Phosphorylation Deficiency: A condition that affects energy production.
Experimental Evidence Supporting Chemiosmosis
- pH Gradient Detection: Experiments have measured pH differences across membranes during electron transport.
- ATP Synthase Function: Isolated enzyme has been shown to produce ATP in response to proton flow.
- Artificial Vesicles: Lab models recreate chemiosmosis with liposomes and proton gradients.
Inhibitors of Chemiosmosis
Certain substances can block chemiosmosis:
- Cyanide: Inhibits complex IV of the ETC.
- Oligomycin: Blocks ATP synthase.
- Uncouplers: Disrupt proton gradient (e.g., DNP).
These compounds help in scientific research and medical diagnostics, but they are often toxic.
Evolutionary Significance of Chemiosmosis
Chemiosmosis likely evolved early in the history of life. The similarity of ATP synthase across species supports a common evolutionary origin. The process is conserved in:
- Bacteria
- Archaea
- Eukaryotes
This universality underlines its essential role in cellular energy management and evolutionary success.
Conclusion
Chemiosmosis is a vital, conserved biological mechanism that enables efficient energy conversion across all domains of life. Whether powering cell division, muscle contraction, or plant growth, it is at the core of bioenergetics. Understanding chemiosmosis not only helps us grasp how life works at the molecular level but also opens doors to innovation in medicine, agriculture, and sustainable energy.
As research advances, our knowledge of chemiosmosis continues to illuminate the elegant design of life’s energy systems and offers potential solutions to some of humanity’s biggest challenges.
Frequently Asked Questions (FAQs)
What is Chemiosmosis?
Chemiosmosis is a process that helps cells make energy. It works by moving tiny particles called protons (H⁺) across a membrane inside the cell. This movement creates power, which the cell uses to make ATP the main energy source your body uses for everything like walking, thinking, or even breathing.
What is the Process of Chemiosmosis?
In chemiosmosis, electrons move through a chain of proteins (called the electron transport chain), which pushes protons across a membrane. This builds up pressure, like water behind a dam. When the protons flow back through a special enzyme called ATP synthase, energy is released and that energy is used to make ATP.
Does Chemiosmosis Require Oxygen?
Yes, chemiosmosis usually needs oxygen during cellular respiration. Oxygen is the final electron acceptor it grabs the electrons and combines with protons to form water. Without oxygen, the process stops, and cells can’t make enough energy to function properly. However, in some bacteria, chemiosmosis can happen without oxygen using different molecules.
Related Articles