Calcofluor White staining is an important fluorescent staining technique extensively used in clinical microbiology, mycology, and histology for the detection of fungal elements, certain parasites, and components of connective tissue. The stain binds specifically to certain carbohydrate polymers present in the cell walls of fungi and other organisms, making it an effective diagnostic and research tool. This method is valued for its rapidity, sensitivity, and ability to visualize microorganisms that may not be easily seen using conventional light microscopy techniques.
Summary of Calcofluor White Staining
- Calcofluor White is a fluorescent dye that binds to chitin and cellulose in fungal cell walls and certain parasitic cysts, making them appear bright blue or green under a fluorescence microscope.
- It provides rapid, sensitive, and high-contrast detection of fungal elements in various clinical and environmental samples, offering results within minutes.
- Though simple and effective, it requires fluorescence microscopy and cannot identify fungal species, necessitating further tests for complete organism identification.
Table of Contents
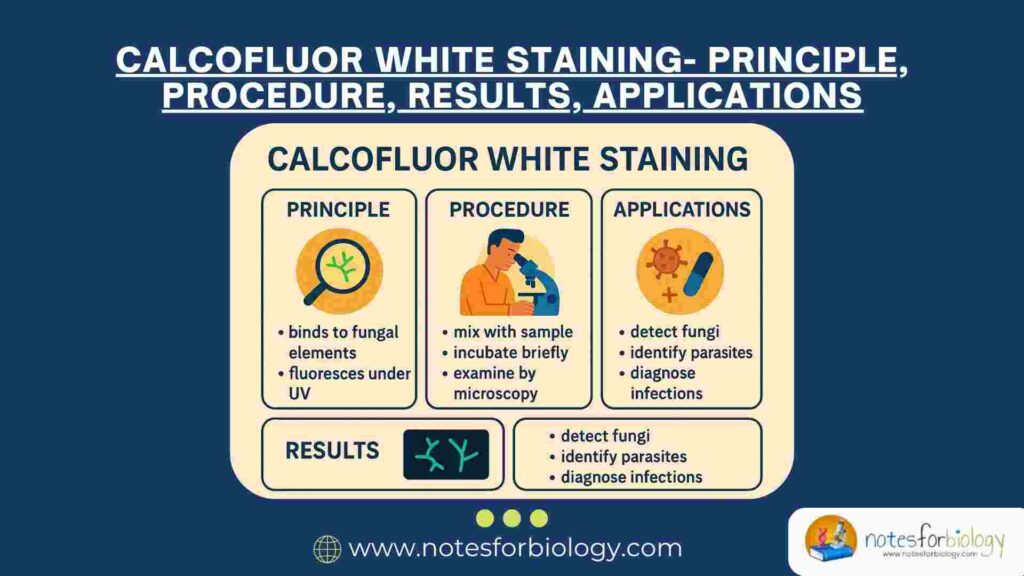
Definition of Calcofluor White Staining
Calcofluor White staining is a fluorescence-based staining method that utilizes Calcofluor White dye, a fluorochrome that binds specifically to polysaccharides containing β-linked glucosyl residues, such as chitin and cellulose. When exposed to ultraviolet (UV) or violet-blue light under a fluorescence microscope, the bound dye fluoresces, allowing clear visualization of fungal cell walls and other structures containing such carbohydrates.
Principle of Calcofluor White Staining
Understanding how Calcofluor White functions is essential to appreciating its diagnostic value. The principle of this staining method is based on the specific binding of the dye to β-linked polysaccharides found in certain biological materials.
Binding to Cell Wall Polysaccharides
Calcofluor White dye selectively binds to chitin and cellulose, which are structural polysaccharides found in fungal cell walls and plant materials. These carbohydrates contain β(1→3) and β(1→4) linkages that have a high affinity for the dye molecules.
Fluorescence Under UV Light
When exposed to ultraviolet or violet-blue light under a fluorescence microscope, the bound dye molecules emit bright bluish-white or greenish fluorescence. This emission occurs due to a change in the dye’s molecular structure upon binding to the polysaccharides.
Enhanced Visualization
As a result of this fluorescence, fungal elements such as hyphae, spores, and yeasts become distinctly visible against a dark background, facilitating easier and quicker detection compared to standard light microscopy.
Reagents Used in Calcofluor White Staining
Calcofluor White Dye
This optical brightener is the key reagent in the staining procedure. It binds specifically to carbohydrate components of cell walls, enabling fluorescence-based detection.
Potassium Hydroxide (KOH)
KOH is commonly used alongside Calcofluor White to dissolve keratin and other background materials in clinical specimens. By clearing the debris, it enhances the visibility of fungal elements.
Evans Blue or Other Counterstains
In certain staining protocols, counterstains like Evans Blue are added to reduce background fluorescence. This results in better contrast between the target structures and the surrounding material.
Specimen Types for Calcofluor White Staining
Calcofluor White staining can be performed on a wide variety of clinical and environmental specimens. Commonly examined clinical materials include skin scrapings, nail clippings, hair shafts, corneal scrapings, sputum, bronchoalveolar lavage, cerebrospinal fluid, blood cultures, and tissue biopsy sections. Environmental samples such as plant material, water samples, and food products can also be evaluated for the presence of fungal contaminants or parasitic cysts using this technique.
Procedure of Calcofluor White Staining
The staining process involves several carefully executed steps to ensure optimal staining quality and accurate results. Each stage contributes to the final visualization under the microscope.
Preparation of the Specimen
The first step in the Calcofluor White staining procedure involves preparing a suitable sample for examination. In the case of skin, hair, or nail samples, small portions are placed on a clean glass slide. Liquid specimens such as cerebrospinal fluid or bronchoalveolar lavage fluid are centrifuged to concentrate the material, and the sediment is transferred onto a slide.
Application of Potassium Hydroxide (KOH)
A drop of 10–20% KOH solution is added to the specimen on the slide. This step serves to digest and clear keratinous and other proteinaceous debris present in the sample, making fungal elements more visible. In tissue sections, this step may be omitted or replaced with other clearing agents, depending on the sample type.
Addition of Calcofluor White Stain
After clearing with KOH, a drop of Calcofluor White staining solution is added directly onto the specimen. The specimen is then gently mixed with the dye and covered with a coverslip to prevent air bubbles, which can interfere with microscopic observation.
Incubation Period
The stained preparation is usually allowed to stand at room temperature for about one to two minutes. This brief incubation period enables sufficient binding of the dye to the target polysaccharide structures within the cell walls.
Examination under a Fluorescence Microscope
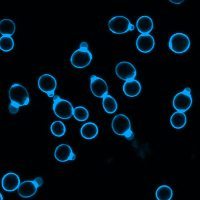
The stained slide is examined under a fluorescence microscope equipped with an appropriate UV or violet-blue excitation filter. Fungal elements and other stained structures fluoresce with a bright bluish-white or apple-green appearance, depending on the filter and dye formulation used, against a dark, non-fluorescing background.
Results of Calcofluor White Staining
Upon microscopic examination, fungal elements such as hyphae, spores, conidia, and yeast cells appear as bright, fluorescent structures, typically bluish-white or greenish under UV illumination. The intensity of fluorescence depends on the concentration of chitin and other β-glucans in the fungal cell wall, as well as the thickness and maturity of the fungal structures.
In addition to fungi, other organisms possessing cellulose-containing cyst walls, such as certain protozoa including Acanthamoeba cysts, also display positive fluorescence. Non-target materials, including human tissue debris, keratin, and inflammatory cells, may show minimal or no fluorescence, which improves the contrast and clarity of the observation.
Applications of Calcofluor White Staining
The versatility of Calcofluor White staining makes it applicable across various disciplines in microbiology, pathology, and environmental studies. Its ability to quickly detect fungal and parasitic organisms is particularly valuable in clinical settings.
Clinical Diagnosis of Fungal Infections
Calcofluor White staining is a valuable diagnostic tool in clinical microbiology for the rapid detection of superficial and deep fungal infections. It is commonly used to identify dermatophytes, yeasts, and opportunistic molds in clinical specimens such as skin scrapings, nail clippings, hair shafts, sputum, bronchoalveolar lavage, cerebrospinal fluid, and tissue biopsies. Its high sensitivity and rapid results make it especially useful in diagnosing life-threatening invasive fungal infections.
Identification of Parasitic Cysts and Oocysts
Apart from fungi, Calcofluor White staining can be employed to detect certain protozoan cysts and oocysts, particularly those with cellulose-based walls. Organisms like Acanthamoeba cysts and Cyclospora oocysts fluoresce under the stain, facilitating their detection in clinical specimens such as stool, eye scrapings, and cerebrospinal fluid.
Examination of Plant and Environmental Samples
In plant pathology and environmental microbiology, Calcofluor White staining is used to identify fungal contaminants in plant tissues, food products, and water samples. Its ability to detect chitin and cellulose makes it suitable for identifying fungal spoilage in agricultural products and microbial contamination in industrial settings.
Histopathological Applications
In histology and pathology laboratories, Calcofluor White staining assists in the examination of tissue sections for the presence of fungal elements. It provides a rapid, sensitive alternative to conventional staining methods like Periodic Acid-Schiff (PAS) and Gomori methenamine silver (GMS), especially in frozen sections or cytological smears.
Advantages of Calcofluor White Staining
Calcofluor White staining offers several distinct benefits that make it a favored method for the detection of fungi and certain parasitic cysts. Its diagnostic value lies in its rapidity, sensitivity, and simplicity. The following points explain the primary advantages of this technique in more detail.
Rapid Diagnostic Turnaround
One of the most significant advantages of Calcofluor White staining is the speed at which results can be obtained. Unlike traditional culture methods, which may require several days to weeks for fungal growth and identification, this staining method yields visible results within minutes. This rapid turnaround is especially valuable in clinical settings where prompt diagnosis is essential for initiating appropriate treatment, particularly in life-threatening systemic fungal infections.
High Sensitivity and Detection of Low Burden Infections
Calcofluor White staining exhibits a high degree of sensitivity in detecting even minimal amounts of fungal elements in clinical specimens. Its ability to bind specifically to chitin and other β-linked polysaccharides ensures that faint or sparse fungal hyphae, spores, or yeast cells are clearly visible under fluorescence microscopy. This feature makes it particularly effective for diagnosing early-stage infections or low burden cases that might be missed by routine staining techniques.
Excellent Contrast and Visualization
The fluorescence produced by Calcofluor White provides a stark contrast between the brightly fluorescing fungal structures and the dark, non-fluorescing background. This high contrast facilitates clear, unambiguous visualization of fungal elements, making identification straightforward even for less experienced observers. The technique is especially useful when examining keratinized tissues or dense clinical material where conventional light microscopy might be obstructed by background debris.
Versatile Application in Various Specimen Types
Calcofluor White staining can be applied to a wide range of clinical and environmental samples. It is effective for detecting fungal infections in skin scrapings, nail clippings, corneal scrapings, sputum, bronchoalveolar lavage, cerebrospinal fluid, and tissue biopsies. In addition, it is useful in detecting parasitic cysts, environmental fungal contamination, and plant pathogens. This versatility makes it a valuable multipurpose tool in both diagnostic and research laboratories.
Simple Technique with Minimal Reagent Requirements
The procedure for Calcofluor White staining is relatively simple and does not demand complex or expensive reagents. The primary requirement is the Calcofluor White dye itself, often used in combination with potassium hydroxide or a counterstain such as Evans Blue. The straightforward technique allows even moderately equipped laboratories to perform the stain efficiently, provided they have access to a fluorescence microscope.
Limitations of Calcofluor White Staining
While the advantages of Calcofluor White staining are considerable, the method is not without its limitations. Certain constraints and potential drawbacks may affect its diagnostic utility in specific settings or for particular organisms. The following subheadings elaborate on the main limitations associated with this technique.
Dependence on Fluorescence Microscopy
A major limitation of Calcofluor White staining is its reliance on fluorescence microscopy for visualization. Fluorescence microscopes are costly, require specialized maintenance, and may not be readily available in smaller or resource-limited healthcare facilities and laboratories. This equipment dependency can restrict the widespread implementation of the technique, especially in low-income or rural settings.
Lack of Species-Level Identification
Although Calcofluor White staining effectively highlights fungal elements and certain parasitic cysts, it does not offer species-level identification. The stain merely confirms the presence of structures containing chitin or cellulose but does not differentiate between species of fungi or protozoa. As a result, additional diagnostic tests such as fungal culture, molecular techniques, or biochemical assays are necessary for definitive organism identification and antifungal susceptibility testing.
Risk of Non-Specific Background Fluorescence
In some cases, non-target materials such as keratin debris, connective tissue components, or improperly cleared background material can exhibit non-specific fluorescence. This background staining may obscure the field of view or create artifacts, complicating interpretation. The problem can be mitigated by careful specimen preparation, effective clearing with potassium hydroxide, and the use of counterstains like Evans Blue to suppress background fluorescence.
Possibility of Photobleaching
Another limitation is the phenomenon of photobleaching, where prolonged exposure to UV or violet-blue light during microscopic examination leads to a gradual loss of fluorescence intensity in stained structures. This fading effect can impair the clarity of observation and potentially result in missed detections if the stained slide is not examined promptly or if it requires extended viewing. To minimize this, slides should be examined immediately after staining and exposure to intense illumination should be limited.
Limited Diagnostic Value in Absence of Chitin or Cellulose
Calcofluor White binds specifically to β-linked polysaccharides such as chitin and cellulose. Consequently, it has no diagnostic value for organisms lacking these structural components. Bacteria, viruses, and many protozoa without cellulose-based cyst walls do not fluoresce with this stain. As a result, the technique is restricted in scope to certain fungi, parasitic cysts, and plant pathogens, limiting its broader microbiological applicability.
Conclusion
Calcofluor White staining is a reliable, rapid, and sensitive fluorescence-based technique employed extensively in clinical and environmental microbiology for the detection of fungal elements and certain parasitic cysts. Its ability to bind selectively to β-linked polysaccharides in cell walls enables clear visualization of pathogens under a fluorescence microscope. With applications ranging from clinical diagnosis to environmental monitoring and histopathological examination, Calcofluor White staining remains an indispensable tool in modern laboratory practice. Understanding its principle, procedure, interpretation, and limitations is essential for microbiologists, pathologists, and laboratory personnel involved in infectious disease diagnostics.
Frequently Asked Questions (FAQ)
What is the purpose of the Calcofluor white stain?
The purpose of Calcofluor White stain is to rapidly detect fungi and certain parasites by binding to their cell wall polysaccharides, making them fluoresce under UV light for easy visualization.
What is a Calcofluor white fungal infection?
A Calcofluor White fungal infection refers to a fungal infection diagnosed using Calcofluor White stain, which highlights fungal elements in clinical samples by causing them to fluoresce.
What is the use of Calcofluor white in clinical mycology?
In clinical mycology, Calcofluor White is used to quickly identify fungal infections in patient samples by staining fungal cell walls, allowing rapid and sensitive microscopic detection.
Related Contents
Classification of Virus – Necessity, Modern System and Importance