Beer-Lambert Law, often known as Beer’s Law, is an important principle in spectroscopy, particularly absorption spectroscopy. It describes the relationship between a substance’s concentration in a solution and its ability to absorb light.
Table of Contents
What is Beer-Lambert Law?
Beer-Lambert Law, commonly known as Beer’s Law, is a key principle in spectroscopy, especially absorption spectroscopy. It describes the link between a substance’s concentration in solution and the amount of light absorbed by the solution at a given wavelength.
In essence, it asserts that a solution’s absorbance (A) is proportional to both the concentration (c) of the absorbing component and the sample’s route length. It can be mathematically represented as follows:
𝐴 = 𝜀 ⋅ 𝑐 ⋅ 𝑙 A=ε⋅c⋅l
Where:
𝐴, A is the absorbance,
𝑐 ,c is the concentration of the absorbing species (in mol/L or g/L),
𝑙 ,l is the sample route length (usually measured in centimeters),
𝜀 ,ε (epsilon) is the molar absorptivity or extinction coefficient.
Derivation
Beer-Lambert Law can be derived from the fundamental laws of light’s interaction with matter. When monochromatic light passes through a solution containing an absorbing species, some of it is absorbed by the species and the rest flows through the solution. The solution’s absorbance is directly proportional to the amount of light absorbed, which is related to the concentration of the absorbing species and the sample’s path length.
Mathematically, the relationship between absorbance, concentration, and path length can be calculated from the Beer-Lambert Law as follows:
1. Start with the definition of absorbance:
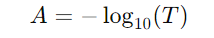
where, T is the transmittance of the solution.
2. Transmittance is the ratio of light intensity transmitted through a sample (𝐼) to incident light intensity (I0)
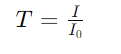
3. The absorbance can be expressed in terms of transmittance:
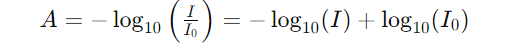
4. The relationship between absorbance and concentration (𝑐) can be represented as follows:
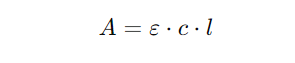
5. Adding equations (3) and (4), we get:
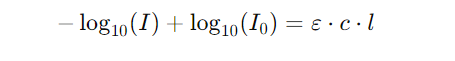
6. Rearranging terms yields the Beer-Lambert Law equation:
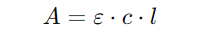
Limitations
Linearity:
The Beer-Lambert Law applies exclusively to a specific concentration range. At extremely high concentrations, the law may no longer be linear due to self-absorption or departures from Beer’s Law
Wavelength Dependence:
The molar absorptivity (𝜀ε) depends on the wavelength of light used for measurement. Different wavelengths of light may interact differently with the absorbing species, resulting in changes in apparent absorbance.
Interference:
Other chemicals in the solution may absorb light of the same wavelength as the analyte, resulting in interference and erroneous readings
Solvent Effects:
Changes in the solvent environment, such as changes in pH or ionic strength, can impact the solution’s absorbance and cause concentration determination mistakes.
Sample Homogeneity:
This assumes that the absorbing species is uniformly distributed throughout the sample. Inhomogeneity or precipitation of the analyte can lead to inaccurate measurements.
Frequently Asked Question(FAQ)
What is Beer-Lambert Law?
Beer-Lambert Law, commonly known as Beer’s Law, is a key principle in spectroscopy, especially absorption spectroscopy. It describes the link between a substance’s concentration in solution and the amount of light absorbed by the solution at a given wavelength.
What are the Limitations of Beer-Lambert Law?
The limitations of Beer-Lambert Law are:
1. Linearity
2. Wavelength Dependence
3. Interference
4. Sample Homogeneity
Related Article