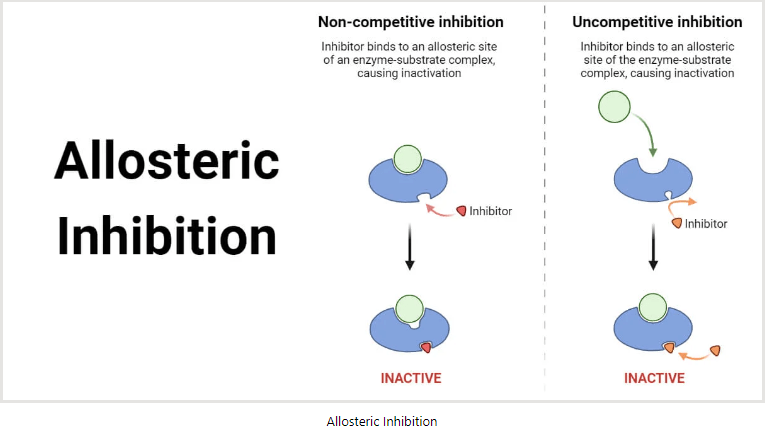
An allosteric inhibitor is a chemical that binds to the enzyme at an allosteric location. The allosteric site differs from the active site in several ways. When the enzyme combines with the inhibitor, its three-dimensional structure is altered. One type of non-competitive inhibition is allostery.
Table of Contents
What is Allosteric inhibition?
Allosteric inhibition is a regulatory mechanism where an inhibitor attaches to an enzyme at a location other than the active site (the allosteric site), changing the enzyme’s shape and lowering its activity.
Mechanism
The mechanism of allosteric inhibition involves several steps, through which the binding of an inhibitor to an allosteric site leads to a decrease in the enzyme’s catalytic activity. Here’s a detailed explanation of this process:
1. Allosteric Inhibitor Binding
The allosteric site is a particular regulatory region on the enzyme that the inhibitor molecule attaches to. The active site of the enzyme, where substrate binding and catalysis take place, is different from this location.
2. Induction of Conformational Change
The structure of the enzyme changes conformationally when the inhibitor binds to the allosteric site. Depending on the particular enzyme and inhibitor involved, this structural modification can impact the enzyme in a number of different ways.
3. Transmission of Structural Changes:
The enzyme undergoes a conformational change that results in modifications to the active site’s dynamics or structure. Changes in the tertiary structure of the enzyme, or its overall three-dimensional form, or quaternary structure, which refers to how numerous subunits are arranged in multi-subunit enzymes, might cause this.
4. Decreased Substrate Affinity or Catalytic Efficiency:
Usually, one or more of the following outcomes arise from the induced conformational change:
Decreased substrate affinity at the active region of the enzyme, changed the orientation of important residues in the active site, reducing the efficiency of the catalytic activity, alteration or obstruction of the active site, which physically prevents the catalytic process or substrate binding.
5. Stabilization of Inactive Form:
In contrast to the active R-state (relaxed state), the T-state (tense state) is an inactive or less active conformation of the enzyme that can occasionally be stabilized by the allosteric inhibitor.
Cooperativity
In the context of allosteric inhibition, cooperativity describes the phenomena wherein the binding affinity of other inhibitor molecules to other allosteric sites on the same enzyme is influenced by the binding of an allosteric inhibitor to one site on the enzyme. This action can lead to positive or negative cooperativity depending on how likely it is that more inhibitor molecules will bind.
1. Positive Cooperativity
When a molecule, such as an allosteric inhibitor, binds to one site on an enzyme, it increases the affinity of other binding sites on the same enzyme for more inhibitor molecules. This phenomenon is known as positive cooperativity in the context of allosteric control. This occurrence is usually the consequence of conformational modifications brought about by the initial binding event, which improve the inhibitor attachment affinity of the remaining binding sites. In many biological processes, positive cooperativity is crucial because it enables a prompt and effective regulatory response, guaranteeing that metabolic pathways.
2. Negative Cooperativity
When a molecule, such as an allosteric inhibitor, binds to one site on an enzyme, the affinity of other sites for subsequent inhibitor molecules is reduced. This process is known as negative cooperativity in allosteric regulation. This happens as a result of the initial binding causing the enzyme to undergo a conformational change that reduces the accessibility or favorability of the remaining binding sites for additional inhibitor binding. This kind of cooperativity ensures that enzymes can react appropriately to different levels of regulatory molecules inside the cell and helps maintain a steady balance of enzyme activity by preventing excessive inhibition.
Examples of Allosteric Inhibition
1. Phosphofructokinase-1 (PFK-1):
- Inhibitors: ATP and citrate.
- Mechanism: When there is an adequate supply of energy, high concentrations of ATP or citrate bind to allosteric sites on PFK-1, resulting in a conformational shift that lowers the enzyme’s affinity for fructose-6-phosphate and downregulates glycolysis.
2. Aspartate Transcarbamoylase (ATCase):
- Inhibitor: CTP (cytidine triphosphate).
- Mechanism: CTP attaches itself to the allosteric site of ATCase, causing a conformational shift that lowers the activity of the enzyme. The synthesis of pyrimidine nucleotides is regulated in part by this feedback inhibition.
3. Hemoglobin:
- Inhibitor: 2,3-Bisphosphoglycerate (2,3-BPG).
- Mechanism: 2,3-BPG attaches itself to an allosteric location in hemoglobin’s central cavity, stabilizing the T-state (deoxy form) and lowering oxygen affinity, which promotes tissue oxygen release.
Summary: Allosteric inhibition is a crucial regulatory mechanism for controlling enzyme activity in response to cellular conditions. Inhibitors provide accurate regulation of metabolic pathways by causing conformational changes that decrease enzyme activity by attaching to an allosteric site.
Frequently Asked Questions:
What do you mean by allosteric inhibition?
Allosteric inhibition is a regulatory mechanism where an inhibitor attaches to an enzyme at a location other than the active site (the allosteric site), changing the enzyme’s shape and lowering its activity.
What are the two types of allosteric inhibitors?
The two main categories of allosteric inhibitors are homotropic and heterotropic inhibitors. Molecules that are identical to the substrate of the enzyme are known as homotropic allosteric inhibitors. Heterotropic allosteric inhibitors, on the other hand, bind to different allosteric sites on the enzyme and differ from the substrate.
Related Articles: