Introduction
Enzymes are special proteins in living organisms that act like catalysts, which means they speed up chemical reactions without being used up themselves. They are very important for carrying out essential functions in the body, such as breaking down food during digestion, producing energy from nutrients, and allowing cells to communicate with each other. However, the activity of enzymes needs to be carefully controlled. If enzymes are too active or not active enough, the balance inside the body can be disturbed, leading to problems.
To help regulate enzyme activity, the body uses molecules known as enzyme inhibitors. These inhibitors slow down or stop the action of specific enzymes. Scientists also use inhibitors to create medicines that treat infections, cancers, and other diseases. Understanding how enzyme inhibitors work helps doctors, researchers, and students learn how the body functions and how diseases can be treated more effectively.
In this extended guide, we will take a deep look into enzyme inhibitors, focusing on three main types: competitive, noncompetitive, and end-product inhibition. We’ll explain how each one works, give real-life examples, and describe why they are important in health, science, and daily life.
Table of Contents
What Are Enzyme Inhibitors?
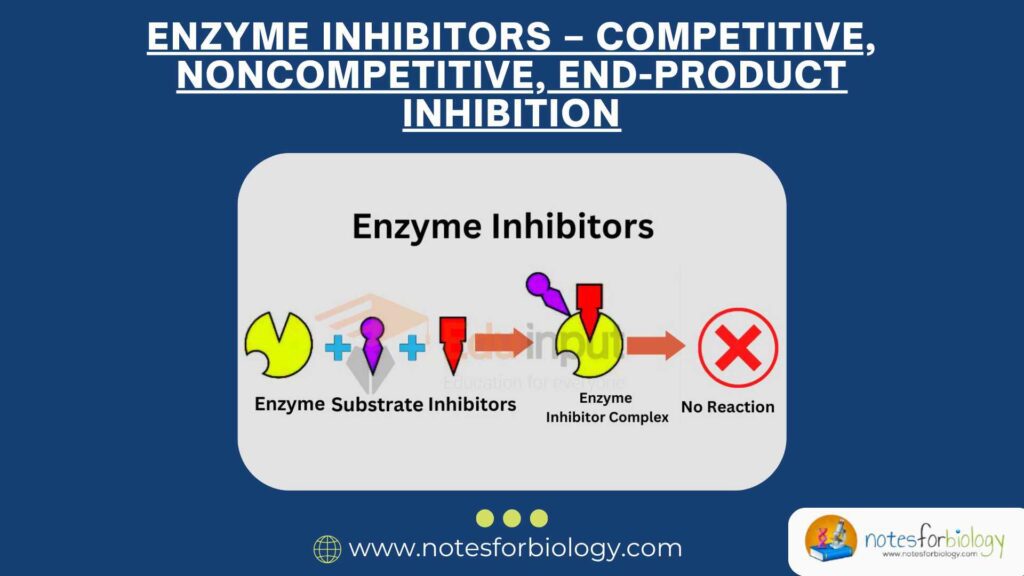
Enzyme inhibitors are substances or molecules that reduce or completely stop the activity of enzymes. When an enzyme is active, it helps speed up a chemical reaction by interacting with a molecule called a substrate. The substrate fits into a special part of the enzyme called the active site, like a key fitting into a lock. But when an inhibitor is present, it either blocks this active site or changes the shape of the enzyme so the substrate cannot fit properly.
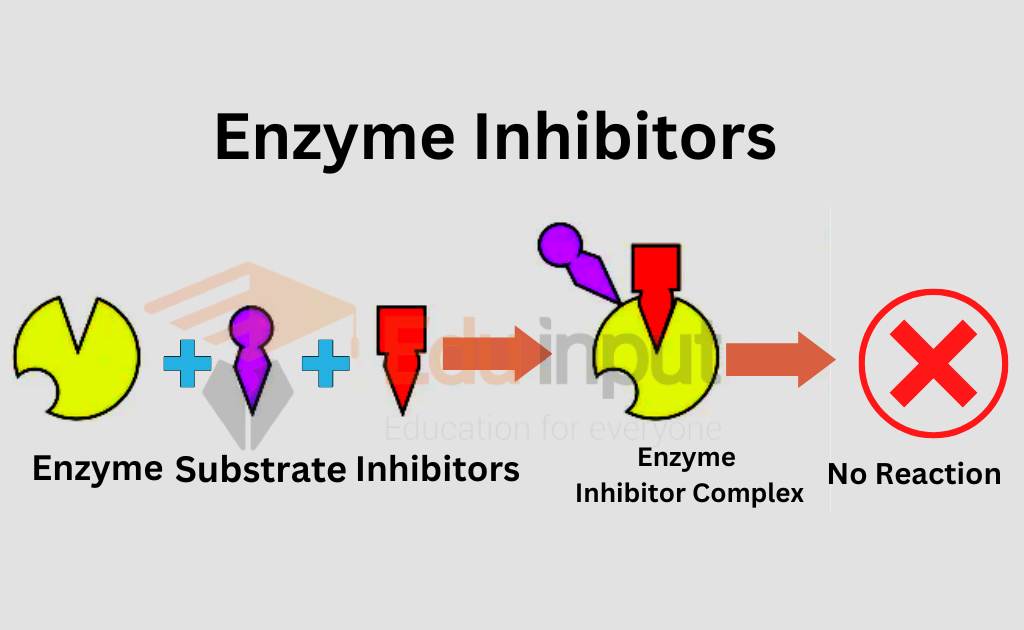
Enzyme inhibitors can be divided into two main types:
- Reversible inhibitors: These bind temporarily to the enzyme and can let go after some time, allowing the enzyme to function again.
- Irreversible inhibitors: These form a permanent bond with the enzyme, completely shutting down its activity for good.
By affecting how enzymes work, inhibitors help control the speed and timing of important chemical reactions in cells.
Competitive Inhibition
Definition
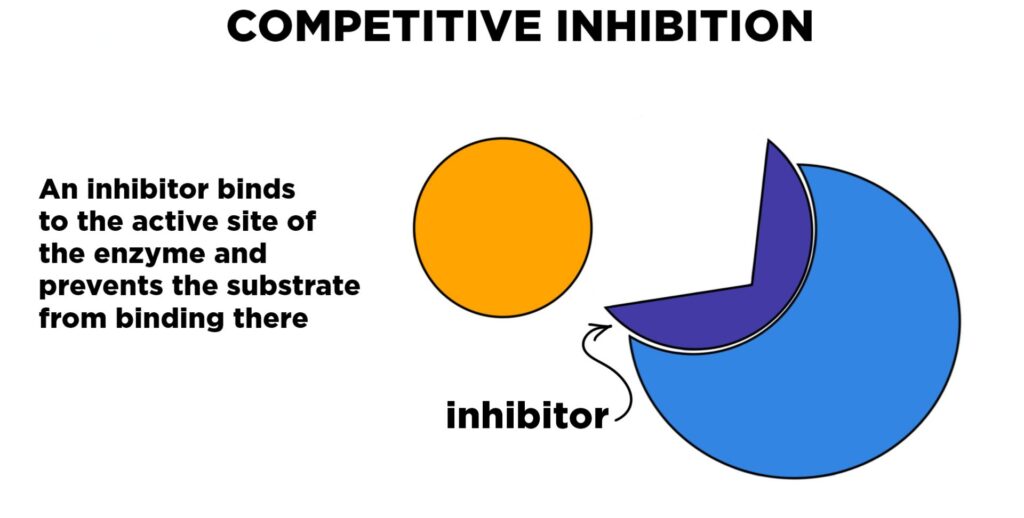
Competitive inhibition is a type of enzyme inhibition where the inhibitor molecule looks very similar to the natural substrate of the enzyme. Because of this similarity, the inhibitor tries to enter the active site of the enzyme, where the real substrate would normally bind. Since both the inhibitor and the substrate are trying to bind to the same spot, they “compete” with each other. This means that if more inhibitor is present, it reduces the chance for the substrate to bind and slows down the reaction.
Mechanism
Here’s how competitive inhibition works:
- This enzyme inhibitor has a similar shape or structure as the enzyme’s natural substrate.
- It fits into the active site of the enzyme, blocking the real substrate from entering.
- As long as the inhibitor is bound, the enzyme cannot carry out its job.
- If the concentration of substrate is increased, the substrate can “outnumber” the inhibitor and bind more often, reducing the effect of the inhibition.
So, the more substrate you add, the more likely it is to win the competition with the inhibitor.
Example
- Malonate and Succinate: In the Krebs cycle, succinate is broken down by an enzyme called succinate dehydrogenase. Malonate is a molecule that looks like succinate and acts as a competitive inhibitor. It fits into the active site but does not react, blocking the enzyme.
- Methotrexate: A drug used to treat cancer and some autoimmune diseases. It resembles folic acid and competes with it to block an enzyme needed for DNA production.
Graphical Representation
When scientists study how enzymes work, they often use graphs. In a Lineweaver-Burk plot, competitive inhibition shows:
- The maximum speed of the reaction (Vmax) stays the same.
- The enzyme seems to have a lower affinity for the substrate (Km increases).
Real-Life Applications
- Medicine: Many drugs are designed as competitive inhibitors to block enzymes that cause disease.
- Research: Scientists use competitive inhibitors to understand how enzymes function.
- Poisoning treatment: Some antidotes are competitive inhibitors that block toxic substances from binding to enzymes.
Noncompetitive Inhibition
Definition
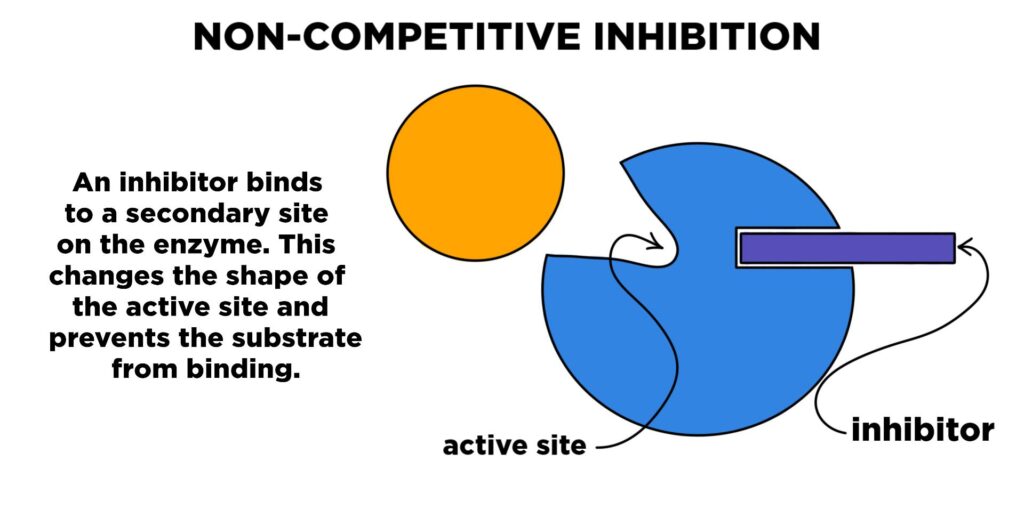
Noncompetitive inhibition is different from competitive inhibition. Instead of competing for the active site, this Enzyme Inhibitors binds to another part of the enzyme called an allosteric site. This binding changes the shape of the enzyme in such a way that even if the substrate can still bind to the active site, the enzyme cannot carry out the reaction effectively.
Mechanism
Here is how noncompetitive inhibition works:
- The inhibitor attaches to a site on the enzyme that is not the active site.
- This causes a change in the enzyme’s shape.
- Even if the substrate binds to the active site, the enzyme can no longer catalyze the reaction properly.
- Adding more substrate does not overcome the inhibition because the problem is with the enzyme’s shape, not with access to the active site.
Example
- Heavy Metals: Metals like lead and mercury can bind to enzymes and permanently change their shape, making them nonfunctional.
- Cyanide Poisoning: Cyanide binds to an enzyme in the mitochondria (cytochrome c oxidase), blocking the body’s ability to produce energy.
Graphical Representation
In a Lineweaver-Burk plot:
- The maximum speed of the reaction (Vmax) is lowered.
- The enzyme’s affinity for the substrate (Km) remains the same.
Real-Life Applications
- Toxicology: Understanding how poisons work and how to treat them.
- Drug development: Some medications are designed as noncompetitive inhibitors.
- Agriculture: Some pesticides work by noncompetitively inhibiting important enzymes in pests.
End-Product Inhibition (Feedback Inhibition)
Definition
End-product inhibition, also known as feedback inhibition, is a way that cells naturally regulate chemical reactions. In this case, the final product of a series of enzyme reactions acts as an inhibitor of one of the first enzymes in the pathway. This helps prevent the overproduction of substances and saves the cell’s resources.
Mechanism
- A metabolic pathway includes many enzymes working in sequence.
- As more of the final product is made, it begins to accumulate.
- This product binds to an enzyme at the beginning of the pathway.
- The enzyme changes shape or becomes inactive, stopping the production of more product.
This is a safe and smart way for cells to prevent waste.
Example
- Isoleucine Production: In bacteria, the amino acid isoleucine is made from threonine. When isoleucine builds up, it inhibits the first enzyme (threonine deaminase), stopping further production.
- ATP Feedback: When a cell has enough energy in the form of ATP, it slows down the enzymes in the energy production pathway.
Graphical Representation
Since many enzymes are involved, graphs for feedback inhibition are more complex. However, the overall effect is a slower rate of production when the final product builds up.
Real-Life Applications
- Biotechnology: Scientists can design pathways that avoid feedback inhibition to increase product yields.
- Health: Helps maintain balance in hormones and nutrients.
- Nutrition and Diet: Understanding how the body controls production of substances like cholesterol.
Comparing the Three Types
| Feature | Competitive Inhibition | Noncompetitive Inhibition | End-Product Inhibition |
|---|---|---|---|
| Where it binds | Active site | Allosteric site | Regulatory site or early enzyme |
| Reversible? | Yes | Yes or no | Yes |
| Can more substrate fix it? | Yes | No | No |
| Real-world examples | Methotrexate, malonate | Cyanide, mercury | Isoleucine synthesis |
| Used in | Drugs, antidotes | Poisons, medicines | Cell regulation |
Importance in Medicine and Research
Understanding enzyme inhibition has helped scientists develop better treatments for many diseases. Examples include:
- Antibiotics: Some block bacterial enzymes, killing harmful bacteria.
- Antiviral drugs: Medications like those used for HIV stop virus enzymes from working.
- Cancer drugs: Target enzymes that help cancer cells grow.
- Painkillers: Medicines like aspirin block enzymes that cause inflammation and pain.
- Heart medicines: Statins lower cholesterol by inhibiting a key enzyme in cholesterol synthesis.
Enzyme Inhibitors in Daily Life
You might not realize it, but enzyme inhibitors are all around you:
- Food preservatives: Some stop enzymes that cause spoilage.
- Cleaning products: Some enzyme blockers protect fabrics or improve cleaning.
- Gardening: Many weed killers block enzymes in plants to stop their growth.
Challenges and Side Effects
While enzyme inhibitors are useful, they can also cause problems if not used carefully:
- Side effects: Medicines that block human enzymes too strongly can cause unwanted effects.
- Resistance: Bacteria and viruses can change to avoid being affected by inhibitors.
- Environmental risks: Some pesticides can harm helpful organisms by blocking their enzymes.
Conclusion
Enzyme inhibitors play a vital role in controlling chemical reactions in the body and in the world around us. The three main types—competitive, noncompetitive, and end-product inhibition—each have unique features that help regulate enzyme activity. They are used in medicine, agriculture, and industry and help researchers understand how life works at a molecular level.
With continued research, enzyme inhibitors will remain a powerful tool in fighting disease, improving health, and creating new technologies.
Three Key Summary of Enzyme Inhibitors
- Enzyme inhibitors slow down or stop enzyme activity, helping regulate important chemical reactions.
- Competitive inhibitors block the active site; noncompetitive inhibitors change the enzyme’s shape; end-product inhibitors act as feedback to shut down a pathway.
- These inhibitors are useful in medicine, industry, agriculture, and scientific research.
Frequently Asked Questions
What is an enzyme inhibitor in simple words?
An enzyme inhibitor is something that stops or slows down an enzyme from doing its job.
Are enzyme inhibitors harmful?
Some are harmful, like poisons, but many are helpful and used as medicines.
Can enzyme inhibition be reversed?
Yes, some inhibitors can be removed, and the enzyme will work again.
Related Articles