Introduction
Electron Spin Resonance (ESR), also known as Electron Paramagnetic Resonance (EPR), is a powerful analytical technique used to study materials that contain unpaired electrons. It is particularly useful in chemistry, physics, biology, and materials science for investigating the behavior and properties of free radicals, transition metal complexes, and defects in solids.
Unlike Nuclear Magnetic Resonance (NMR), which detects the spin of atomic nuclei, ESR specifically detects the magnetic moments of electrons. The ESR technique is based on the principle that unpaired electrons in an external magnetic field absorb microwave radiation at a characteristic frequency, allowing their detection and analysis.
In this document, we will explore the principle of ESR, the components of its instrumentation, the method of operation, and the wide-ranging applications of this fascinating technique all in a clear, humanized, and easy-to-understand format.
Table of Contents
What is Electron Spin Resonance (ESR)?
Electron Spin Resonance (ESR) is a spectroscopic technique that detects species with unpaired electrons, such as:
- Free radicals
- Transition metal ions
- Defect centers in crystals
- Reactive intermediates in chemical reactions
These species have magnetic properties due to the presence of an unpaired electron, and ESR makes it possible to study their structure, environment, and dynamics.
Basic Concept of Electron Spin
Every electron has two types of angular momentum:
- Orbital angular momentum (due to its motion around the nucleus)
- Spin angular momentum (an intrinsic property, like a tiny bar magnet)
Electrons have a spin quantum number of ±½. This spin creates a magnetic moment, which interacts with external magnetic fields.
When an electron is placed in a magnetic field, its spin states split into two energy levels:
- Lower energy state (aligned with the field)
- Higher energy state (opposed to the field)
This energy difference forms the basis of ESR spectroscopy.
Principle of ESR
The principle of Electron Spin Resonance (ESR) lies in the resonant absorption of microwave radiation by unpaired electrons in an external magnetic field.
How it works:
- An external magnetic field is applied to a sample containing unpaired electrons.
- The magnetic field causes the electron spin states to split (Zeeman splitting).
- Microwave radiation of a specific frequency is directed at the sample.
- When the energy of the microwaves matches the energy gap between the spin states, electrons absorb the radiation and transition to the higher energy state.
- This absorption is detected and plotted as an ESR spectrum.
The basic equation governing ESR is:
ΔE = hν = gβB
Where:
- ΔE = energy difference between spin states
- h = Planck’s constant
- ν = frequency of microwave radiation
- g = g-factor (proportionality constant specific to the electron’s environment)
- β = Bohr magneton
- B = applied magnetic field
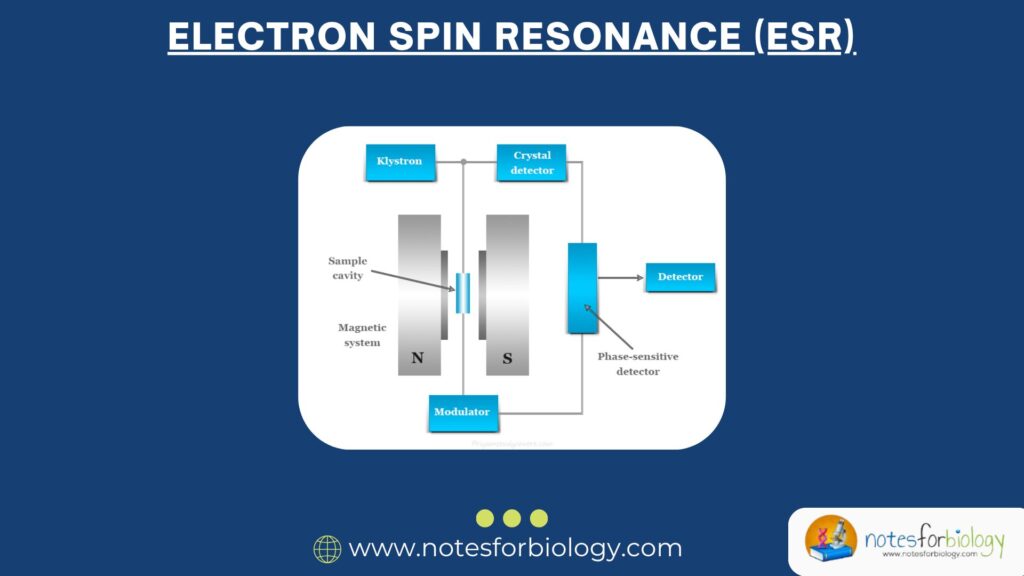
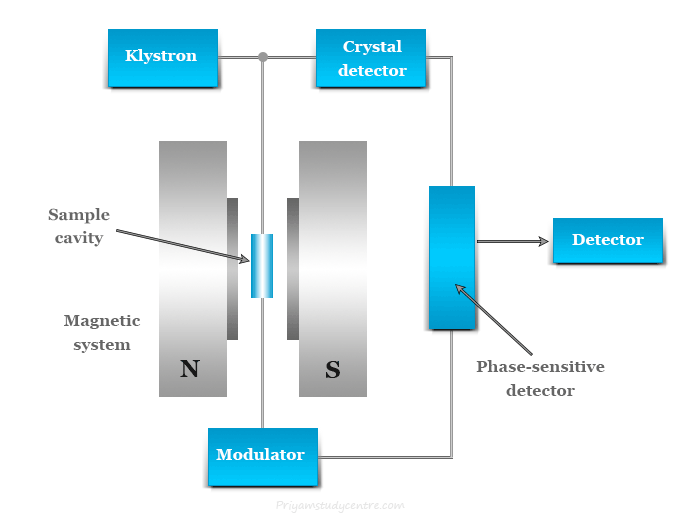
Components of Electron Spin Resonance (ESR) Instrumentation
ESR instruments are designed to detect and amplify the microwave absorption signal from unpaired electrons. A typical ESR spectrometer includes the following components:
1. Microwave Source
- Generates microwave radiation (usually around 9–10 GHz for X-band ESR)
- Commonly a klystron oscillator
2. Sample Holder (Resonant Cavity)
- Holds the sample within the magnetic field
- Amplifies the signal through resonance
3. Magnet
- Provides a stable and variable magnetic field (up to several thousand gauss)
- Often includes electromagnets or superconducting magnets
4. Modulation Coils
- Modulate the magnetic field to improve sensitivity and resolution
- Typically operated at 100 kHz
5. Detector System
- Measures the intensity of absorbed microwave radiation
- Uses diode detectors or crystal detectors
6. Display and Recording Unit
- Converts the detected signal into a visual plot or spectrum
- Usually a computer interface with graphical software
7. Cooling System (Optional)
Liquid nitrogen or helium may be used to cool the sample and enhance signal clarity, especially for unstable radicals
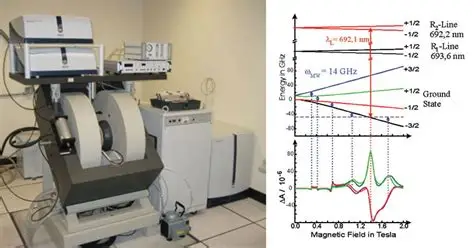
Electron Spin Resonance (ESR) Spectrum and Interpretation
An ESR spectrum typically shows one or more peaks, each representing a resonant absorption event. The position, number, shape, and intensity of these peaks reveal detailed information about the sample.
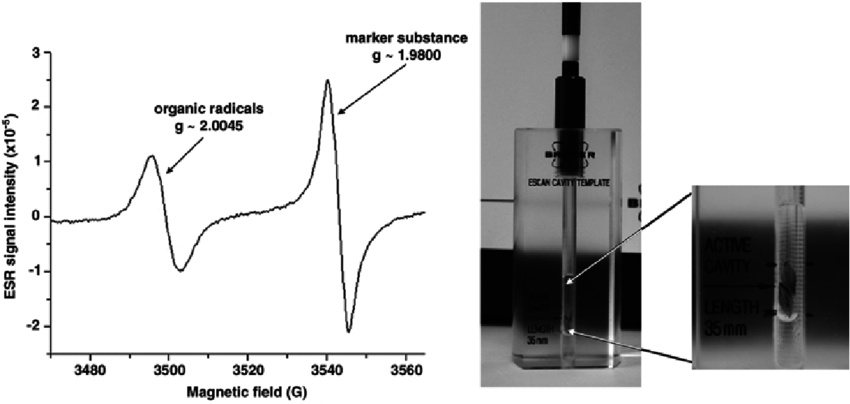
Key parameters in Electron Spin Resonance (ESR) spectrum:
1. g-factor
- Indicates the magnetic environment of the electron
- Deviations from the free electron g-value (2.0023) reflect the chemical environment
2. Hyperfine Splitting
- Interaction between unpaired electrons and nearby atomic nuclei (with non-zero nuclear spin)
- Results in splitting of ESR signals into multiple lines
3. Line Width
- Reflects the stability and relaxation of the electron spin system
- Broad lines may indicate fast relaxation or solid-phase samples
4. Signal Intensity
- Proportional to the number of unpaired electrons in the sample
- Can be used for quantitative analysis
Types of Electron Spin Resonance (ESR) Techniques
Different ESR techniques and modifications allow for a variety of analyses:
1. Continuous Wave ESR (CW-ESR)
- Most common form
- Sample is exposed to continuous microwave radiation while sweeping the magnetic field
2. Pulsed ESR
- Involves short bursts of microwave pulses
- Used to study dynamic processes and measure relaxation times (T1 and T2)
3. Electron-Nuclear Double Resonance (ENDOR)
- Combines ESR and NMR
- Provides enhanced structural and electronic information
4. High-Frequency ESR (HF-ESR)
- Uses higher microwave frequencies (>95 GHz)
- Increases resolution and sensitivity
5. Time-Resolved ESR (TR-ESR)
- Observes transient species formed during fast reactions
- Useful in photochemistry and radical kinetics
Applications of Electron Spin Resonance (ESR)
ESR has a wide range of applications in different scientific fields. Some of the key applications are listed below:
In Chemistry:
1. Free Radical Detection
- Identification and characterization of free radicals in chemical reactions
- Monitoring radical intermediates in polymerization, combustion, and photochemical processes
2. Reaction Mechanism Studies
Understanding pathways and kinetics of chemical reactions
3. Redox Reactions
Investigating oxidation and reduction behavior of metal ions
In Biology and Medicine:
1. Biomedical Research
- Study of reactive oxygen species (ROS) and nitric oxide radicals in cells
- Understanding oxidative stress and its role in aging, cancer, and neurodegenerative diseases
2. Radiation Biology
- Assessing free radical damage caused by ionizing radiation
- Dosimetry using ESR signal intensity in irradiated materials (like teeth or bones)
3. Drug Development
Studying interaction of drugs with free radicals and enzymes
In Material Science:
1. Defect Analysis
Detecting and characterizing lattice defects in crystals and semiconductors
2. Catalysis
Investigating the active sites of metal catalysts
3. Conducting Polymers
Analysis of charge carriers (polarons, bipolarons) in organic materials
In Environmental Science:
1. Pollution Monitoring
Detection of pollutant-derived radicals in air, water, and soil
2. Soil Chemistry
Investigating paramagnetic centers in minerals
In Archaeology and Geology:
1. Dating of Fossils and Rocks
ESR can estimate the age of materials by measuring trapped electrons
2. Paleodose Measurement
Measures absorbed radiation dose over time in archaeological samples
Advantages of Electron Spin Resonance (ESR)
- Highly specific to unpaired electrons
- Non-destructive technique
- Provides both qualitative and quantitative data
- Can detect short-lived species (with modifications)
- Useful across multiple scientific disciplines
Limitations of ESR
- Only applicable to paramagnetic species (not diamagnetic)
- Complex spectra may require advanced interpretation
- Sensitivity may be lower than some other methods
- Requires specialized equipment and trained personnel
Safety Considerations
- Handle high magnetic fields and microwave radiation with care
- Use shielding to prevent exposure
- Cryogens like liquid nitrogen must be handled with proper PPE
Conclusion
Electron Spin Resonance (ESR) is an incredibly useful spectroscopic technique that allows scientists to probe the otherwise invisible world of unpaired electrons. By applying magnetic fields and microwave radiation, ESR reveals details about molecular structure, chemical environment, and reaction mechanisms.
Whether it’s identifying harmful radicals in a biological system, dating ancient fossils, or designing better materials, ESR plays a crucial role in advancing science. Its versatility and precision make it a powerful tool for researchers across disciplines.
Three Key Summary
- ESR detects unpaired electrons using microwave radiation in the presence of a magnetic field.
- It provides valuable insights into free radicals, metal complexes, and structural defects.
- Widely used in chemistry, biology, physics, materials science, and environmental monitoring.
Frequently Asked Questions (FAQs)
What type of samples can be analyzed by ESR?
ESR is best suited for samples that contain unpaired electrons, such as free radicals, transition metal ions, and paramagnetic defects.
What is the difference between ESR and NMR?
ESR detects electrons while NMR detects nuclei. ESR is more sensitive to paramagnetic species.
Why is ESR not as commonly used as NMR?
Because ESR only works on paramagnetic species, its applications are more specialized compared to NMR.
Related Articles