Chromatography is a foundational analytical technique that has become indispensable across science and industry. It enables precise separation, identification, and purification of complex mixtures, supporting advancements in pharmaceuticals, environmental analysis, food chemistry, and forensic science. As innovations unfold, it continues to evolve, maintaining its position at the forefront of analytical tools.
Its roots trace back to the early 20th century when Russian botanist Mikhail Tsvet first separated plant pigments, laying the groundwork for modern chromatography. Since then, the field has expanded dramatically with the introduction of new stationary phases, detectors, and automation that have enhanced sensitivity, speed, and reproducibility.
Table of Contents
What is Chromatography?
It is a method that separates a mixture’s components based on their interactions with two phases: a stationary phase and a mobile phase. This fundamental principle underlies a wide range of analytical techniques, from thin‐layer plates to advanced mass spectrometry coupling. Chromatography makes it possible to isolate, detect, and analyze compounds present at extremely low concentrations.
The term “chromatography” derives from the Greek words “chroma” (color) and “graphein” (to write), reflecting its early use in separating and identifying colored compounds in plant extracts. Today, modern chromatography routinely works with colorless and complex molecules, ranging from small organics to large biomolecules, with impressive specificity and resolution.
Role of Stationary and Mobile Phases
In chromatographic separations, the stationary phase remains fixed while the mobile phase moves through it, carrying the sample components. Components that interact more strongly with the stationary phase move slowly, while those favoring the mobile phase travel faster. This interaction disparity enables effective separation based on chemical properties and affinities.
The nature and design of both phases such as polarity, particle size, and pore structure strongly influence chromatographic behavior. Careful selection of phase materials and buffer conditions allows chemists to tailor separations for specific target molecules, whether small analytes or large proteins.
Separation Based on Differential Partitioning
It relies on the principle that different substances partition between the mobile and stationary phases based on their physicochemical characteristics. Compounds that are more soluble in the mobile phase tend to elute quickly, whereas those with higher affinity for the stationary phase are retained. This differential partitioning produces distinct peaks or spots, representing separated components.
Successful separations depend on optimizing these partitioning differences; small changes in temperature, solvent composition, or column chemistry can significantly impact resolution. Controlled manipulation of these parameters enables selective separation for compounds with similar chemical properties.
Factors Affecting Separation Efficiency
Effective chromatographic separations require consideration of various factors including flow rate, temperature, column length, and the nature of the analytes. Shorter columns and faster flow speeds can reduce analysis time but may compromise resolution. Conversely, longer columns with optimal packing and moderate flow rates achieve high separation efficiency, measured by the number of theoretical plates.
Additional variables such as mobile phase viscosity, injection volume, and sample load also influence band broadening and peak shape. Addressing each aspect systematically ensures robust and reproducible separations.
Types
Its methods can be classified by the physical state of the mobile phase and by the mechanism through which separation occurs. This organizational framework helps guide method selection based on analyte characteristics and analytical requirements.
Classification Based on Physical State
Gas Chromatography (GC) uses an inert gas mobile phase, making it ideal for analyzing volatile and thermally stable compounds. It provides rapid and high-resolution separations.
Liquid Chromatography (LC) employs a liquid mobile phase and is suitable for a broad range of analytes including polar, non-volatile, and thermally labile compounds. It offers versatility in column and solvent choices.
Supercritical Fluid Chromatography (SFC) uses supercritical CO₂ or other fluids, combining the best aspects of GC and LC. It offers fast, efficient separations with reduced solvent consumption and environmental impact.
Classification Based on Separation Mechanism
Adsorption Chromatography separates compounds based on differential adsorption to a solid stationary phase like silica or alumina. It’s widely used in preparative purification and analytical separation.
Partition Chromatography separates analytes based on their partitioning between a liquid coated onto a solid and the mobile phase; reversed-phase LC is a common form suited for a wide range of molecules.
Ion Exchange Chromatography relies on electrostatic interactions between charged analytes and an oppositely charged stationary phase; it’s commonly used in protein and nucleic acid purification.
Size Exclusion (Gel Filtration) Chromatography separates molecules based on size by passing them through a porous matrix; smaller molecules are retained longer than larger ones.
Affinity Chromatography exploits highly specific biological interactions—such as antibody-antigen or receptor-ligand binding—for selective purification and enrichment of target molecules.
Detailed Overview of Major Types
Each chromatography type offers unique strengths tailored to specific analytical and preparative needs, allowing chemists to select the most appropriate method based on sample and target analyte properties.
Gas Chromatography (GC)
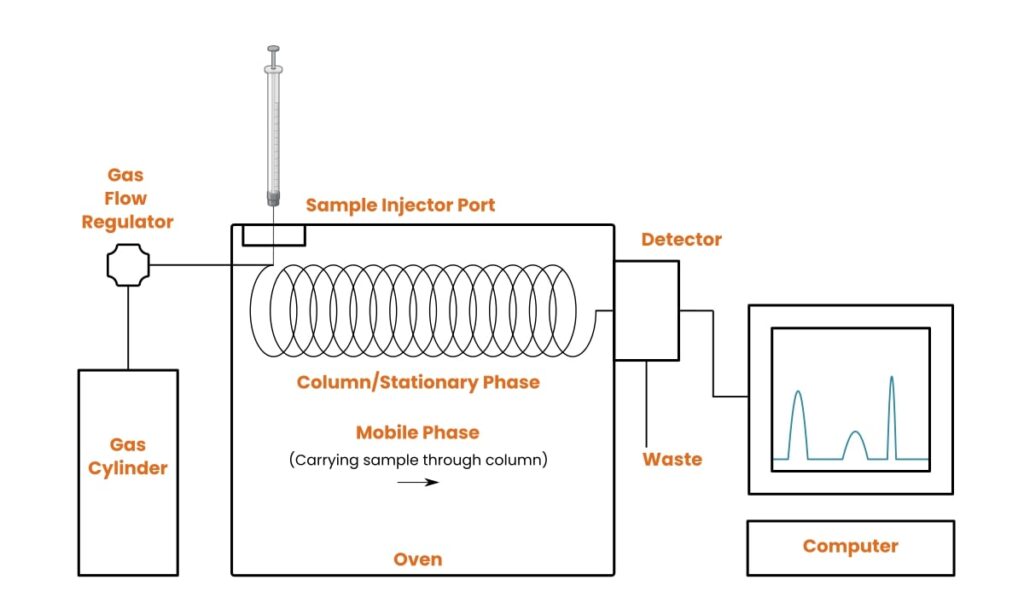
Principle and Instrumentation
GC separates volatile molecules in a heated column packed with a stationary phase, usually capillary in structure. An inert gas carries the sample, and detectors such as FID or mass spectrometers identify and quantify the eluted compounds with high sensitivity.
Applications in Environmental Testing and Forensics
GC is often used to monitor air and water pollutants such as pesticides, VOCs, and industrial contaminants. In forensic laboratories, GC helps analyze blood alcohol levels, drug residues, and accelerants, providing strong evidence in legal investigations.
Liquid Chromatography (LC)
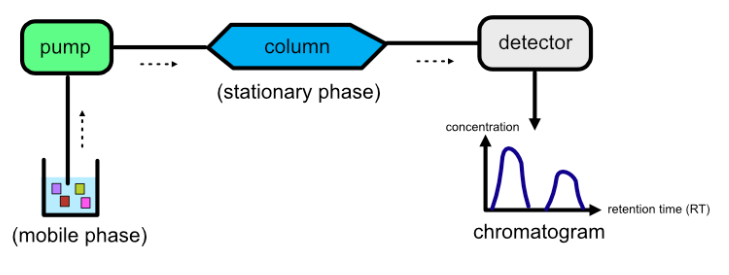
High-Performance Liquid Chromatography (HPLC)
HPLC uses high-pressure pumps to force solvents through a packed column, delivering precise and reproducible separations. It can analyze complex mixtures such as pharmaceuticals, biological fluids, and food components with high resolution.
Ultra-High-Performance Liquid Chromatography (UHPLC)
UHPLC utilizes smaller particle sizes and higher pressures, reducing analysis time while enhancing efficiency and sensitivity. It is widely employed in pharmaceutical development and proteomics.
Applications in Pharmaceuticals and Biochemistry
LC is essential for drug discovery, impurity testing, pharmacokinetic studies, and biomolecule quantification. It enables monitoring of therapeutic compounds, metabolites, proteins, and peptides at trace levels in complex biological matrices.
Thin Layer Chromatography (TLC)
Basic Procedure and Materials Used
TLC is performed on a plate coated with a thin layer of silica or alumina. Samples are spotted near the base and developed with a mobile-phase solvent. Separated spots appear as distinct bands, detected by UV light or chemical reagents.
Common Analytical Applications
TLC is quick, affordable, and ideal for preliminary screening, reaction monitoring, and compound verification. It’s commonly employed in synthetic chemistry labs and quality check routines.
Paper Chromatography
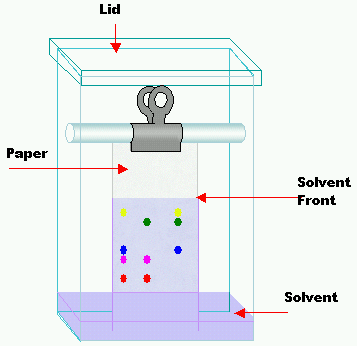
Setup and Working Principle
Paper chromatography uses cellulose paper and an appropriate solvent whereby components separate based on solubility and capillary action. Pigments and small polar molecules separate visibly for qualitative analysis.
Educational and Laboratory Uses
Its simplicity and visual clarity make it a staple in educational settings for demonstrating chemical principles and phase partitioning. It is also used in biochemical laboratories for analyzing amino acids and small peptides.
Column Chromatography
Techniques and Stationary Phase Selection
In column chromatography, a mobile phase flows through a column packed with silica, alumina, or resin. Fraction collection allows separation based on compound polarity, size, or other properties. The choice of packing material and solvent system directly influences the effectiveness of the separation.
Organic Chemistry Applications
This technique is central in research labs for purifying organic synthesis products, separating stereoisomers, and isolating natural products. Gradients or solvent variations enhance selectivity and yield.
Applications
It’s adaptability makes it invaluable in a wide array of industries, providing critical data and purity assurance from research through production.
Pharmaceutical Industry
In drug development, chromatography is used for analysis of drug candidates, impurity profiling, stability testing, and determining pharmacokinetic properties supporting regulatory compliance and patient safety.
Environmental Analysis
Robust methods detect and quantify pollutants at trace levels to ensure compliance with environmental regulations. Examples include pesticides in groundwater and persistent organic pollutants in soil.
Food and Beverage Testing
Used to monitor pesticide residues, detect food additives, analyze flavor compounds, and authenticate sources. Chromatography verifies product safety and consistency before market distribution.
Forensic Science
It assists in analyzing blood alcohol, illicit drugs, arson accelerants, and toxic substances. These results support legal proceedings and criminal investigations.
Clinical and Biomedical Research
Techniques such as LC-MS and GC-MS are used in metabolomics, proteomics, hormone assays, and therapeutic drug monitoring, contributing to diagnostics, personalized medicine, and biomarker discovery.
Petrochemical Industry
Chromatographic analyses are critical for fuel composition, quality control, impurity evaluation, and additive performance in petrochemical and refining operations, enhancing safety and efficiency.
Advantages and Limitations
It offers unmatched resolution and versatility, making it a mainstay in analytical laboratories. However, it is not without challenges that require careful consideration.
Key Benefits of Chromatographic Techniques
It provides high sensitivity, precise separation, and flexibility in handling a diverse range of analytes. It supports both qualitative and quantitative analysis with reproducible and reliable results.
Common Challenges and How to Overcome Them
Limitations include high equipment and operating costs, method development complexity, sample preprocessing needs, and reliance on organic solvents. Advances in automation, miniaturization, and greener solvent alternatives are addressing many of these challenges.
Recent Innovations
It continues to advance through new technologies aimed at improving performance and sustainability while expanding analytical capabilities.
Hyphenated Techniques: GC‑MS, LC‑MS
Integrating it with mass spectrometry enhances both qualitative and quantitative analyses, enabling precise identification even in complex or trace-level contexts.
Automation and High‑Throughput Chromatography
Modern systems equipped with autosamplers, multiplexed column configurations, and robotic handlers support rapid, unattended analysis boosting efficiency in pharmaceuticals and environmental labs.
Green Chromatography and Eco‑Friendly Practices
Efforts to minimize solvent usage, recover mobile phases, and adopt biodegradable materials are gaining momentum. Supercritical CO₂ and aqueous-based methods are becoming increasingly common.
Ethical and Safety Considerations
Effective chromatography demands careful attention to ethical and safety protocols. Handling hazardous solvents and high-pressure systems requires skilled operation and regulatory adherence to ensure user and environmental protection.
Proper waste management, personal protective equipment, and good laboratory practices are essential to reduce chemical hazards and maintain integrity. Compliance with global standards ensures trust and responsibility in analytical results.
Conclusion
It remains an exceptionally versatile and powerful technique in analytical science. Its ability to separate and analyze compounds with remarkable precision makes it indispensable in research, quality control, and diagnostics.
As the field evolves with advancements in instrumentation, green chemistry, and automation, it will continue to enable new discoveries. By embracing innovation and sustainability, chemists can continue to rely on it as a tool for solving pressing scientific and industrial challenges.
Frequently Asked Questions (FAQ)
What are the main components of a chromatography system?
A chromatography system typically includes a mobile phase, stationary phase, sample injector, separation column, detector, and data recorder or processor.
How does chromatography differ from other separation techniques?
Chromatography separates components based on their differential distribution between a mobile and stationary phase, while other techniques like filtration or centrifugation rely on physical properties like size or density.
What is the difference between HPLC and GC?
HPLC uses a liquid mobile phase and is suitable for non-volatile, thermally unstable compounds, while GC uses a gaseous mobile phase and is ideal for volatile, thermally stable substances.
Related Contents
Cholesterol Structure & Synthesis: Pathways, Functions, and Clinical Importance
Chlorophyll- Definition, Structure, Types, Biosynthesis, Uses