Introduction
A gradient refers to a gradual change or difference in quantity or value between two points in space. It tells us how something changes over a distance—this “something” could be temperature, pressure, concentration, height, or even color intensity. In the world of biology, chemistry, and even physics, one concept plays a crucial role in how substances move and interact—concentration gradient. You’ve probably heard of it in the context of osmosis or diffusion, but its influence goes far beyond that. Understanding this concept can provide deep insights into cellular processes, industrial applications, and even environmental science.
Table of Contents
What is a Concentration Gradient?
Definition
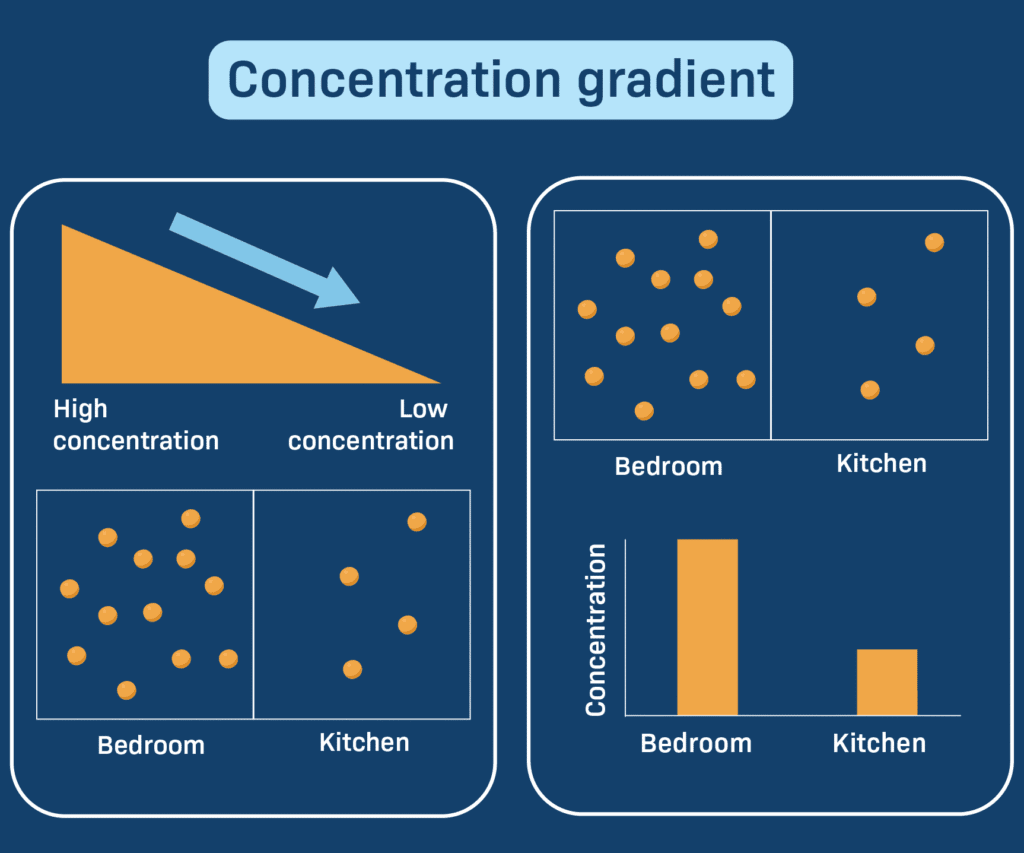
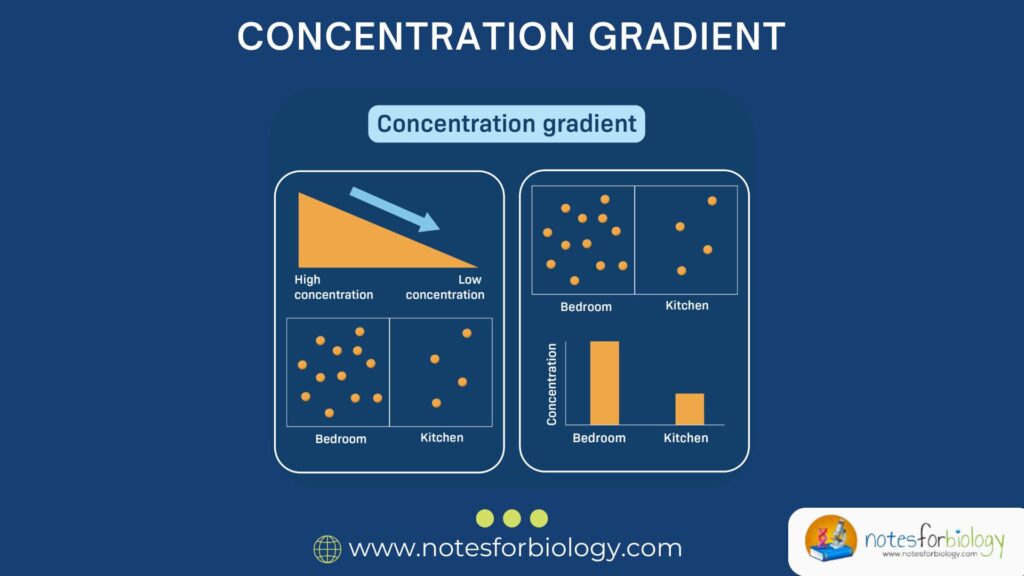
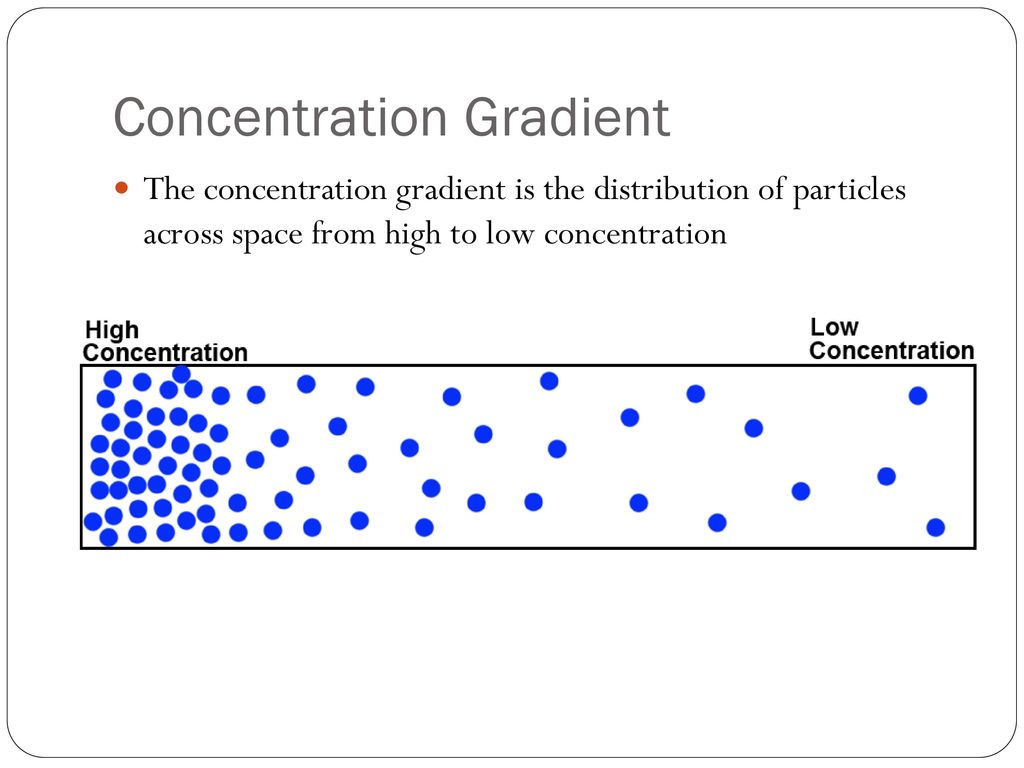
A concentration gradient refers to the gradual difference in the concentration of a substance (like ions, molecules, or gases) between two areas. This difference creates a sort of “chemical slope”—just like a hill—where substances tend to move from a region of higher concentration to a region of lower concentration.
This movement is generally passive, meaning it doesn’t require any additional energy. The process continues until equilibrium is reached, where the concentrations on both sides become equal.
Real-Life Analogy
Imagine spraying perfume in one corner of a room. Initially, the scent is concentrated where it was sprayed. Over time, it spreads throughout the room. That spreading happens because of a concentration gradient—from high concentration near the spray to low concentration in the rest of the room.
Importance of Concentration Gradients
- Cellular function: Cells rely heavily on concentration gradients for exchanging gases, nutrients, and waste.
- Signal transduction: Many signals in the body work through gradient mechanisms.
- Energy production: Gradients help in ATP production via chemiosmosis in mitochondria and chloroplasts.
Types of Movement Across Concentration Gradients
1. Diffusion

The most basic type—particles move from high to low concentration.
2. Facilitated Diffusion
Uses protein channels to help substances move across membranes.
3. Osmosis
Special case of diffusion involving water molecules moving through a semi-permeable membrane.
4. Active Transport
Unlike the others, this requires energy (usually in the form of ATP) to move substances against the gradient—from low to high concentration.
Factors Affecting Concentration Gradients
1. Temperature
Higher temperatures increase the kinetic energy of particles, making them move faster and increasing the rate of diffusion.
2. Molecule Size
Smaller molecules diffuse faster than larger ones.
3. Distance
Shorter distances allow faster movement along the gradient.
4. Surface Area
Larger surface areas allow more molecules to pass through, increasing diffusion rate.
5. Permeability of Membrane
The nature of the membrane (e.g., how many channels or openings it has) affects the ease with which substances move.
6. Concentration Difference
The steeper the gradient (i.e., bigger difference in concentrations), the faster the movement.
Concentration Gradient in Biology
1. Cell Membranes
Cell membranes use concentration gradients to control what enters and exits. Sodium-potassium pumps, for example, maintain a concentration gradient of these ions, which is essential for nerve impulses.
2. Respiration and Photosynthesis
In mitochondria, a proton gradient is used to generate ATP. Similarly, chloroplasts use a gradient to produce energy during photosynthesis.
3. Neurons and Nerve Signals
The movement of ions across neurons, creating electrical signals, depends on ionic concentration gradients.
4. Kidney Function
Your kidneys use concentration gradients to reabsorb water and essential nutrients from urine.
Concentration Gradient in Chemistry
1. Reaction Rates
In chemical reactions, concentration gradients influence how fast reactants convert to products.
2. Chromatography
This technique separates substances based on how they move through a medium, often influenced by concentration gradients.
Concentration Gradient in Medicine
1. Drug Delivery
Medications often rely on gradients to diffuse into cells or across membranes.
2. Oxygen Therapy
Oxygen moves from areas of high concentration (oxygen mask) to low concentration (lungs).
3. Cancer Treatments
Targeted therapies may exploit concentration gradients between tumor cells and surrounding tissues.
Concentration Gradient in Industry
1. Water Purification
Reverse osmosis uses pressure to force water through a membrane against a concentration gradient, removing impurities.
2. Food Preservation
Salt and sugar create gradients that pull water out of microbial cells, preserving food.
3. Chemical Manufacturing
Industrial processes often require precise control of gradients for optimal reaction conditions.
Concentration Gradient and Environmental Science
1. Pollutant Dispersion
Air and water pollution spread via gradients—pollutants move from areas of high concentration to low.
2. Ecosystem Balance
Nutrient gradients affect plant growth and animal distribution.
Graphical Representation of Concentration Gradient
Visualizing a gradient helps in understanding it better. On a graph:
- X-axis: Position or distance
- Y-axis: Concentration
A steep slope shows a strong gradient, while a flat line indicates equilibrium.
Summary Table: Applications of Concentration Gradient
| Field | Application |
|---|---|
| Biology | Cellular transport, nerve signaling |
| Medicine | Drug delivery, oxygen diffusion |
| Industry | Water purification, food preservation |
| Environment | Pollutant spread, ecosystem balance |
Challenges and Limitations
1. Resistance in Movement
Barriers like thick membranes can slow diffusion.
2. Equilibrium State
Once equilibrium is reached, no net movement occurs unless energy is added (like in active transport).
3. Pathological Conditions
Diseases can disrupt natural gradients, affecting processes like gas exchange or fluid balance.
Innovations and Research
- Nanotechnology: Using gradients to guide drug nanoparticles to specific tissues.
- Synthetic Biology: Engineering artificial membranes with tunable gradient controls.
- Climate Research: Studying CO2 gradients for better climate modeling.
Conclusion
The concept of the concentration gradient lies at the heart of many biological, chemical, and physical processes. From the passive movement of molecules during diffusion and osmosis to the intricate mechanisms of active transport, the idea of a substance moving from an area of higher concentration to an area of lower concentration forms the backbone of countless natural and engineered systems.
Understanding the concentration gradient not only helps in explaining cellular behaviors like nutrient absorption, waste removal, and nerve impulse transmission, but also plays a crucial role in medicine (e.g., drug delivery), industrial applications (e.g., dialysis, water purification), and even environmental science (e.g., pollutant dispersion).
The factors affecting concentration gradients—such as temperature, membrane permeability, surface area, and molecular size—remind us how delicate and interconnected these processes are. A small change in one of these parameters can lead to significant shifts in transport efficiency or direction.
In real-world applications, this knowledge is being used to optimize medical treatments, improve biotechnological systems, design better materials, and even create energy-efficient solutions. The movement of ions across membranes in artificial kidneys, the diffusion of gases in respiratory therapy, or the application of concentration gradients in microfluidic devices are just a few examples of how powerful and applicable this principle is.
What makes the concept even more fascinating is its universality—the same principle governs how oxygen moves into a cell, how sugar dissolves in water, and how scent molecules travel through air. In every corner of life and matter, the concentration gradient silently drives the flow, balances systems, and keeps life moving forward.
Ultimately, by deepening our understanding of concentration gradients, we enhance our ability to interpret, predict, and manipulate a vast array of natural and artificial processes. It is not just a scientific concept; it’s a fundamental principle of organization in the universe, demonstrating how imbalance can lead to movement, and how nature constantly seeks equilibrium.
FREQUENTLY ASKED QUESTIONS
What is a concentration gradient?
It’s the difference in the concentration of a substance between two areas.
Why do substances move along concentration gradients?
They move naturally from high to low concentration to achieve equilibrium.
Can substances move against the gradient?
Yes, but it requires energy, as in active transport.
Related Articles