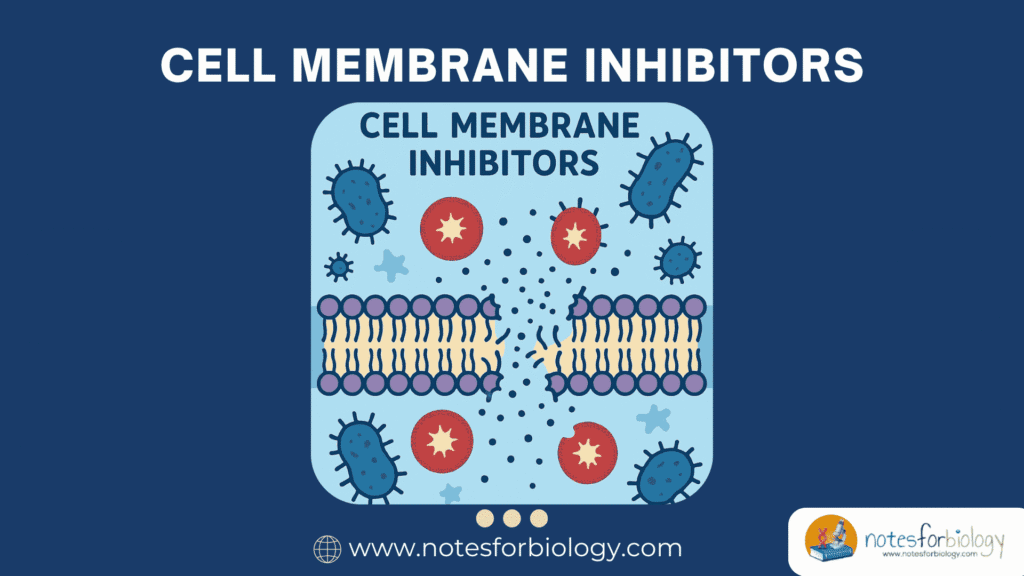
Cell membrane inhibitors are a specialized class of antimicrobial agents that exert their action by targeting the integrity, structure, or function of microbial cell membranes. These inhibitors cause disruption of membrane permeability, leakage of intracellular contents, and eventual cell death.
This mechanism is particularly valuable because the cell membrane is essential for maintaining the ionic balance, nutrient transport, and structural stability of the cell. Disrupting these processes rapidly kills or weakens the microbe, making membrane inhibitors crucial in both clinical and industrial microbiology.
Summary of Cell membrane inhibitors
- Cell membrane inhibitors damage microbial membranes, causing leakage of vital cell contents and rapid cell death.
- Examples include polymyxins, daptomycin, gramicidin, and amphotericin B, used against resistant bacteria and fungi.
- Resistance develops through membrane alterations, efflux pumps, and biofilm formation, reducing drug effectiveness.
Table of Contents
Mechanism of Action of Cell Membrane Inhibitors
The antimicrobial activity of cell membrane inhibitors involves direct interaction with lipid bilayers or membrane proteins, leading to functional collapse. Unlike agents that target nucleic acids or protein synthesis, these inhibitors compromise the physical and chemical barrier provided by the membrane itself.
By integrating into or disturbing the membrane structure, these compounds cause leakage of ions, ATP, nucleotides, and other cytoplasmic constituents. This destabilization impairs critical cellular functions such as respiration, osmoregulation, and signal transduction, often resulting in rapid bactericidal effects.
Examples of Cell Membrane Inhibitors
Several well-known antimicrobial agents fall under this category, differing in their sources, chemical structures, and microbial targets. These compounds are widely used in both clinical medicine and microbiological research.
Polymyxins
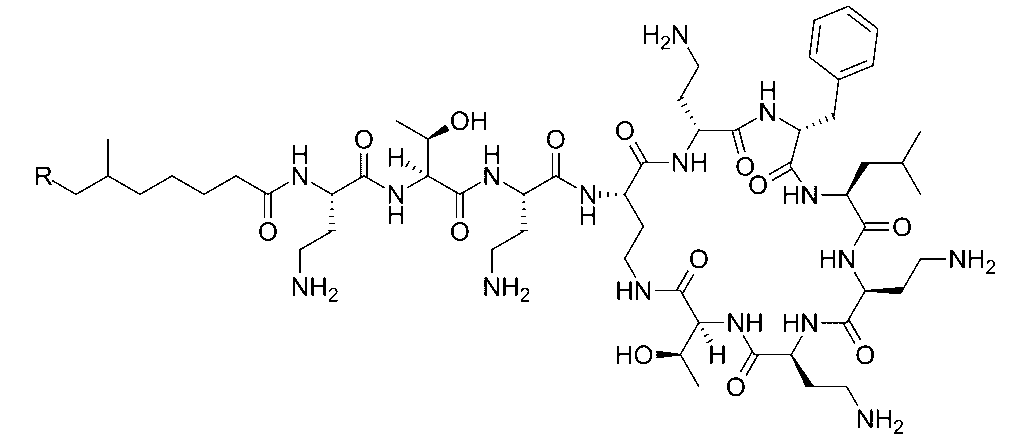
Polymyxins, including Polymyxin B and Polymyxin E (colistin), are cyclic peptide antibiotics produced by Bacillus polymyxa. They bind to the lipid A component of lipopolysaccharides in Gram-negative bacterial membranes, disrupting their integrity.
Polymyxins insert themselves into the phospholipid bilayer, increasing membrane permeability. This leads to leakage of potassium, magnesium, and other ions, culminating in cell lysis. They are especially valuable for treating multi-drug resistant Gram-negative infections.
Daptomycin
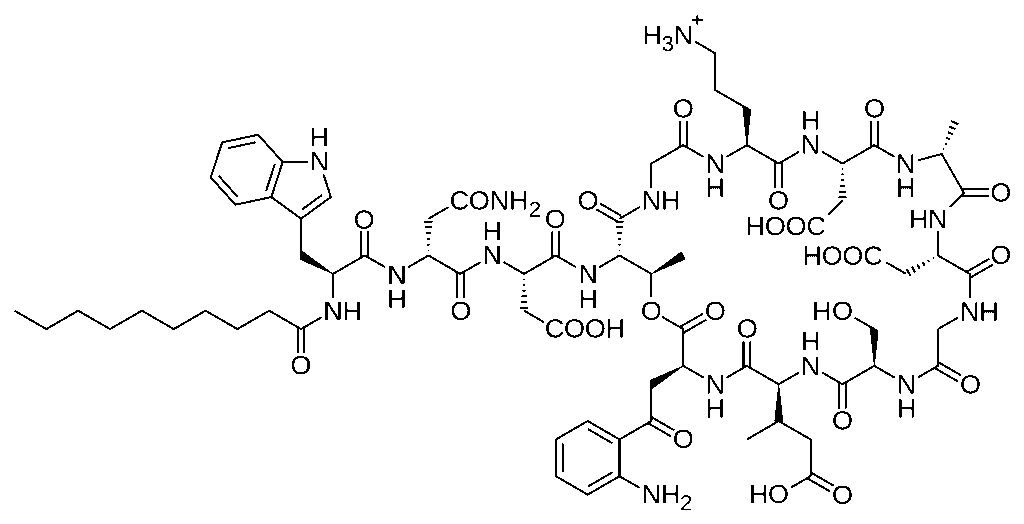
Daptomycin is a lipopeptide antibiotic effective against Gram-positive bacteria. It binds to bacterial membranes in a calcium-dependent manner, forming complexes that create pores in the membrane.
These pores allow efflux of ions, particularly potassium, leading to rapid depolarization and cell death. Daptomycin is especially important for treating drug-resistant infections such as those caused by Staphylococcus aureus and Enterococcus species.
Gramicidin
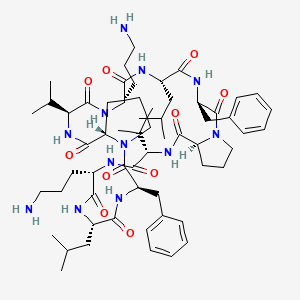
Gramicidin is a linear peptide antibiotic that forms ion channels across the lipid bilayer. It facilitates the uncontrolled passage of monovalent cations like potassium and sodium, disrupting the electrochemical gradient and osmotic balance.
Produced by Bacillus brevis, gramicidin is mainly used topically because of its toxicity to eukaryotic cell membranes. It is a classical example of a pore-forming peptide.
Amphotericin B
Amphotericin B is an antifungal polyene macrolide that binds to ergosterol, a unique fungal membrane sterol. It creates transmembrane pores, allowing leakage of potassium and other small molecules, leading to fungal cell death.
Its selective toxicity toward fungi is based on its higher binding affinity for ergosterol over cholesterol found in mammalian membranes, although some toxicity to human cells still occurs.
Inhibition Effects of Cell Membrane Disruptors
The inhibitory effects produced by these agents are characterized by rapid loss of membrane integrity, which affects multiple downstream cellular processes. Membrane inhibitors often act faster than other antimicrobial classes because they bypass intracellular target sites.
Loss of Membrane Potential
Disruption of ion gradients across the membrane causes a collapse of the membrane potential, an essential component of energy transduction and active transport. As a result, the cell can no longer sustain ATP synthesis via membrane-bound enzymes like ATP synthase.
This also halts nutrient uptake and waste efflux, creating a toxic intracellular environment that accelerates cell death.
Leakage of Cellular Components
Membrane-disrupting antibiotics cause leakage of essential molecules such as nucleotides, amino acids, and electrolytes. The rapid outflow of potassium, in particular, severely disrupts enzyme function and protein synthesis, halting cell growth within minutes.
Loss of cytoplasmic contents is visually confirmed in laboratory settings using techniques such as dye uptake assays or measurement of extracellular protein and nucleic acid concentrations.
Impaired Cellular Respiration
As membranes house components of the electron transport chain (ETC), disruption leads to cessation of aerobic respiration and ATP depletion. In some cases, this also triggers oxidative stress due to accumulation of reactive oxygen species.
The combination of energy failure, osmotic imbalance, and molecular leakage leads to swift bacterial or fungal cell death.
Mechanisms of Resistance to Cell Membrane Inhibitors
Despite their potency, some microbes develop resistance mechanisms that reduce the efficacy of membrane inhibitors. These adaptations primarily involve structural modifications to membrane components or activation of compensatory pathways.
Alteration of Target Molecules
Bacteria may modify membrane lipids or proteins to reduce the binding affinity of antibiotics. For example, Gram-negative bacteria can alter the structure of lipid A in lipopolysaccharides, decreasing polymyxin binding.
Fungal cells, similarly, may decrease ergosterol content or replace it with alternative sterols to lower Amphotericin B interaction, diminishing pore formation and leakage.
Activation of Efflux Pumps
Some microbes acquire or upregulate efflux pump systems that actively expel antimicrobial agents from the cell membrane before they reach lethal concentrations. This strategy is particularly effective for small lipophilic antibiotics like gramicidin.
Efflux pumps not only reduce intracellular antibiotic levels but also contribute to multidrug resistance by transporting structurally unrelated compounds.
Biofilm Formation
Microorganisms embedded within biofilms are naturally more resistant to membrane-disrupting agents due to the protective extracellular polymeric matrix. The biofilm restricts antibiotic penetration, and cells within this community often express stress-response genes that further enhance resistance.
This phenomenon complicates treatment of chronic infections like cystic fibrosis lung infections or catheter-associated bacteremia, where biofilm-embedded bacteria resist even high-dose membrane inhibitors.
Applications of Cell Membrane Inhibitors
The clinical and industrial value of these antimicrobials extends beyond individual patient care, influencing infection control strategies and microbiological safety protocols.
Treatment of Drug-Resistant Infections
Membrane inhibitors like polymyxins and daptomycin are last-resort drugs for multi-drug resistant infections, especially those caused by Acinetobacter baumannii, Pseudomonas aeruginosa, and MRSA. Their rapid bactericidal action is crucial when conventional antibiotics fail.
Such treatments often involve careful monitoring due to potential toxicity, but they remain indispensable in modern medicine.
Antifungal Therapy
Amphotericin B and related compounds are essential in managing systemic fungal infections like cryptococcal meningitis and invasive candidiasis, particularly in immunocompromised individuals. Despite toxicity risks, their effectiveness against resistant fungi is unmatched.
Topical formulations of membrane inhibitors are also used to treat superficial fungal and bacterial infections.
Laboratory Research Tools
Cell membrane inhibitors are valuable in microbiology laboratories for studying membrane physiology, permeability changes, and bioenergetics. Their ability to disrupt specific membrane functions makes them useful probes in experimental setups.
They also serve as positive controls in antibiotic susceptibility assays when evaluating novel antimicrobial candidates.
Conclusion
Cell membrane inhibitors represent a crucial group of antimicrobial agents known for their rapid and direct mechanism of action. By compromising the integrity of microbial membranes, they induce catastrophic leakage of cellular contents and inhibit essential processes like respiration and nutrient transport.
While their potency makes them indispensable for treating drug-resistant infections and systemic mycoses, challenges such as toxicity and emerging resistance highlight the need for careful use and continued research. Their ongoing application in clinical, industrial, and research settings underscores their enduring significance in microbiology.
Frequently Asked Questions (FAQ)
What are cell membrane inhibitors and how do they work?
Cell membrane inhibitors are a type of antimicrobial agent that damage the structural integrity of microbial cell membranes. By disrupting the membrane, they cause leakage of essential ions and molecules, leading to rapid loss of vital functions and cell death.
Which drugs are considered examples of cell membrane inhibitors?
Common examples include polymyxins, which target Gram-negative bacteria; daptomycin, effective against Gram-positive bacteria; gramicidin, which forms ion channels in membranes; and amphotericin B, which targets fungal cell membranes by binding to ergosterol.
How do microorganisms develop resistance against cell membrane inhibitors?
Microorganisms can resist these drugs by altering membrane structures to reduce drug binding, increasing the activity of efflux pumps to expel the drugs, or forming biofilms that act as a protective barrier against antibiotic penetration.