The cell cycle is a fundamental biological process through which cells grow, duplicate their genetic material, and divide to produce new cells. It is essential for growth, development, tissue repair, and maintaining genetic continuity in both prokaryotic and eukaryotic organisms. Understanding the cell cycle’s sequential phases, regulatory mechanisms, and checkpoints is crucial for interpreting how cells maintain orderly division and what happens when these processes are disrupted.
In this explanation, we will explore the definition, distinct phases, regulation strategies, and checkpoints of the cell cycle in an expanded, structured academic format.
Summary of Cell Cycle
- The cell cycle is the orderly series of events that a cell goes through to grow, replicate its DNA, and divide into two identical daughter cells.
- It consists of two main phases: Interphase (G₁, S, G₂) where the cell grows and prepares for division, and the Mitotic phase (M) where the nucleus and cytoplasm divide.
- Proper regulation of the cell cycle is essential for normal growth, tissue repair, and genetic stability. Abnormalities can lead to uncontrolled cell division, mutations, and diseases like cancer.
Table of Contents
Meaning of Cell Cycle
The cell cycle is a series of highly organized, sequential events that a cell undergoes to duplicate its contents and divide into two daughter cells. This cyclical process ensures that each daughter cell inherits an identical and complete copy of the parent cell’s genetic material, along with sufficient cytoplasmic content to function independently.
It is a continuous process but is typically divided into distinct phases based on observable changes in cellular structure, biochemical activity, and DNA content. The orderly nature of the cycle is vital for maintaining tissue integrity, enabling organismal growth, and preventing diseases such as cancer.
Historical Context
The conceptual framework of the cell cycle was established in the late 19th century with the advent of light microscopy. Early biologists observed chromosomal behavior during cell division and classified it into distinct stages. The advent of molecular biology and flow cytometry further refined the understanding of the biochemical and regulatory aspects of the cycle, providing detailed insights into its control systems.
Today, the cell cycle is a key focus in areas like developmental biology, oncology, and regenerative medicine.
Phases of Cell Cycle
The eukaryotic cell cycle is traditionally divided into interphase (the longest phase) and the mitotic (M) phase (where division occurs). Interphase itself is subdivided into G₁, S, and G₂ phases. In certain situations, cells may exit the cycle into a resting state known as G₀.
Interphase
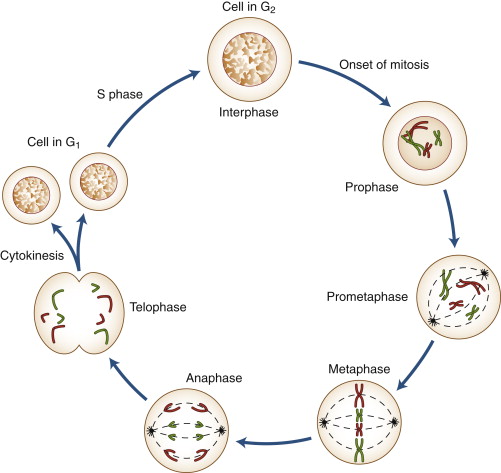
Interphase is the preparatory phase in which the cell grows, duplicates its DNA, and synthesizes the organelles and proteins necessary for division. Though visually less dramatic under the microscope, it involves intense biochemical activity and occupies about 90% of the cell cycle.
Interphase consists of three sequential sub-phases: G₁ (Gap 1), S (Synthesis), and G₂ (Gap 2), each with specialized roles.
G₁ Phase (Gap 1)
In the G₁ phase, the cell grows in size, synthesizes mRNA and proteins, and carries out routine metabolic functions. It prepares the enzymatic and structural materials necessary for DNA replication.
This phase is also crucial for checking the external environment and internal readiness of the cell before committing to DNA synthesis. Cells that fail to meet division criteria may exit the cycle into a quiescent G₀ phase or undergo programmed cell death.
S Phase (Synthesis)
During the S phase, the cell duplicates its entire nuclear DNA, ensuring that each daughter cell receives an identical genetic complement. DNA replication is semi-conservative, involving the unzipping of the double helix and complementary strand synthesis.
Centrosomes, which play a vital role in chromosome separation during mitosis, also duplicate in this phase. Accurate DNA replication is essential to maintain genetic stability.
G₂ Phase (Gap 2)
In the G₂ phase, the cell undergoes a second growth spurt, producing additional proteins and organelles. It verifies the integrity of replicated DNA and repairs errors detected during synthesis.
The phase also prepares structural components like spindle fibers and condenses chromatin, setting the stage for mitosis. Cells that successfully complete G₂ enter the mitotic phase.
Mitotic (M) Phase
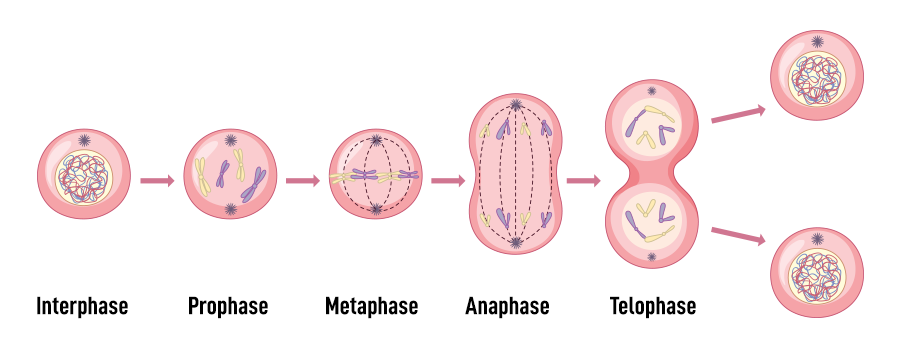
The M phase is where the actual division of the nucleus (karyokinesis) and cytoplasm (cytokinesis) occurs, resulting in two genetically identical daughter cells.
Mitosis itself is further subdivided into prophase, metaphase, anaphase, and telophase, with each stage marked by specific chromosomal and cytoskeletal changes.
Prophase
In prophase, chromatin condenses into visible chromosomes, each consisting of two sister chromatids attached at a centromere. The nuclear envelope begins to break down, and centrosomes move to opposite poles of the cell, initiating spindle fiber formation.
Metaphase
During metaphase, chromosomes align at the cell’s equatorial plane, forming the metaphase plate. Spindle fibers attach to the centromere’s kinetochore, ensuring equal tension and readiness for separation.
Anaphase
In anaphase, the centromeres split, and spindle fibers shorten, pulling sister chromatids toward opposite poles of the cell. This ensures each new nucleus receives an identical set of chromosomes.
Telophase
Telophase marks the reversal of prophase events. Chromosomes decondense back into chromatin, the nuclear envelope reforms around each set of chromosomes, and spindle fibers disassemble.
Cytokinesis
Cytokinesis is the division of the cytoplasm and organelles, typically overlapping with telophase. In animal cells, a contractile ring forms a cleavage furrow that pinches the cell into two. In plant cells, a cell plate forms, eventually becoming the new cell wall separating daughter cells.
Regulation of Cell Cycle
To maintain genomic integrity and proper tissue function, the cell cycle is tightly regulated through complex control systems. These regulatory mechanisms involve proteins and enzymes that ensure the orderly progression of phases and prevent abnormal division.
Cyclins and Cyclin-Dependent Kinases (CDKs)
The primary regulators of the cell cycle are cyclins and cyclin-dependent kinases (CDKs). Cyclins are regulatory proteins whose concentrations fluctuate throughout the cycle, while CDKs are enzymes that, when activated by cyclins, phosphorylate target proteins to advance the cell to the next phase.
Distinct cyclin-CDK complexes operate at different checkpoints, ensuring precise control over DNA replication and division. Their timely synthesis and degradation dictate the orderly transition between phases.
CDK Inhibitors
CDK inhibitors (CKIs) act as negative regulators by binding to cyclin-CDK complexes, blocking their activity when conditions are unfavorable. These inhibitors, such as p21, p27, and p16, enforce checkpoints and can trigger cell cycle arrest to prevent damaged or unprepared cells from dividing.
The balance between CDK activation and inhibition is crucial for healthy cell proliferation and tumor suppression.
Checkpoints of Cell Cycle
Checkpoints are surveillance mechanisms that monitor the integrity of critical events in the cell cycle, such as DNA replication and chromosome segregation. They ensure the cell only progresses when conditions are optimal, and errors are repaired.
G₁ Checkpoint (Restriction Point)
The G₁ checkpoint assesses cell size, nutrient availability, DNA integrity, and extracellular signals. If conditions are favorable, the cell proceeds to the S phase. If damage is detected, cell cycle arrest occurs, allowing for DNA repair or triggering apoptosis.
This is a critical control point for preventing cancer development, as it prevents the replication of damaged DNA.
G₂ Checkpoint
At the G₂ checkpoint, the cell verifies the completeness and accuracy of DNA replication. It checks for unrepaired DNA damage and incomplete replication forks. Only cells with intact and fully duplicated DNA proceed to mitosis.
This checkpoint ensures genetic stability and prevents propagation of mutations.
Metaphase (Spindle Assembly) Checkpoint
The metaphase checkpoint, also called the spindle assembly checkpoint, ensures that all chromosomes are correctly attached to the spindle apparatus at their kinetochores before progressing to anaphase.
Failure of this checkpoint can result in unequal chromosome distribution, leading to aneuploidy or chromosomal instability, often implicated in tumorigenesis.
Abnormalities in the Cell Cycle
While the cell cycle is a highly regulated process designed to maintain order and genetic fidelity, abnormalities can arise when control systems fail. These abnormalities can lead to unchecked cell proliferation, mutations, and the development of diseases such as cancer. Understanding these irregularities provides insight into the mechanisms of cellular malfunction and opens avenues for therapeutic intervention.
Uncontrolled Cell Division
One of the most significant abnormalities in the cell cycle is uncontrolled cell division, where cells bypass regulatory checkpoints and proliferate without restraint. This usually occurs due to mutations in genes that code for cyclins, CDKs, or checkpoint proteins.
Cells with damaged DNA or abnormal chromosomal numbers may continue to divide, resulting in tumor formation. The loss of contact inhibition — where cells normally stop dividing upon reaching a boundary is a hallmark of malignant cells.
DNA Damage and Mutation Accumulation
Failure to repair DNA damage before progressing through the cycle can lead to the accumulation of mutations. These mutations might affect genes responsible for cell cycle regulation, apoptosis, and DNA repair mechanisms.
If such mutations occur in proto-oncogenes (which promote division) or tumor suppressor genes (which inhibit division), they can disrupt normal cycle regulation, leading to carcinogenesis.
Aneuploidy
Aneuploidy refers to an abnormal number of chromosomes resulting from errors in chromosome segregation during mitosis. Faulty spindle formation, kinetochore malfunction, or failure of the metaphase checkpoint can lead to unequal distribution of chromosomes.
Aneuploidy is frequently observed in cancer cells and can contribute to tumor progression, metastasis, and drug resistance.
Significance of the Cell Cycle
The cell cycle is indispensable for various physiological processes in living organisms. Its significance extends beyond mere cell duplication, influencing growth, development, maintenance, and repair.
Growth and Development
The cell cycle facilitates organismal growth by increasing the number of cells. In multicellular organisms, regulated cell division allows tissues and organs to attain proper size and function. During embryogenesis, rapid and precisely timed cell cycles enable the formation of complex body structures from a single fertilized egg.
Tissue Repair and Regeneration
After injury, cells in damaged tissues rapidly re-enter the cell cycle to replace lost or damaged cells. Skin cells, intestinal epithelial cells, and blood cells routinely undergo cell cycles to maintain tissue integrity and function.
Specialized regenerative capacities, such as liver regeneration, also rely on the careful control of the cell cycle in differentiated cells.
Maintenance of Genetic Stability
The orderly progression of the cell cycle ensures that each daughter cell receives an exact copy of the genetic material. The checkpoints and repair mechanisms embedded in the cycle prevent the propagation of DNA errors, safeguarding genetic integrity across generations of cells.
Any disruption in this fidelity may lead to mutations and genetic disorders.
Clinical Relevance of the Cell Cycle
A comprehensive understanding of the cell cycle has immense clinical implications, particularly in diagnosing, treating, and preventing various diseases, including cancer.
Cancer and Cell Cycle Dysregulation
Cancer is fundamentally a disease of uncontrolled cell proliferation caused by disruptions in the cell cycle’s regulatory machinery. Mutations in genes coding for cyclins, CDKs, CDK inhibitors, and checkpoint proteins result in cells dividing unchecked, accumulating mutations, and forming tumors.
Key genes like p53, often dubbed the “guardian of the genome,” play crucial roles in halting the cell cycle in response to DNA damage. Mutations in p53 are implicated in over 50% of human cancers.
Chemotherapy and Radiation Therapy
Many anti-cancer treatments target rapidly dividing cells by interfering with specific phases of the cell cycle. For example:
- Antimetabolites disrupt DNA synthesis in the S phase.
- Mitotic inhibitors block spindle formation in the M phase.
- Radiation therapy induces DNA damage, triggering checkpoints and apoptosis in proliferating tumor cells.
While effective, these treatments can also affect normal rapidly dividing cells, leading to side effects such as hair loss and immunosuppression.
Regenerative Medicine
Cell cycle manipulation is pivotal in regenerative medicine and tissue engineering. Inducing differentiated cells to re-enter the cell cycle and proliferate offers promising strategies for organ regeneration and wound healing.
Techniques like induced pluripotent stem cell (iPSC) technology exploit cell cycle re-entry to produce multipotent cells capable of differentiating into various cell types.
Genetic Disorders
Certain genetic conditions, like ataxia-telangiectasia and Bloom syndrome, result from defects in genes responsible for cell cycle checkpoints and DNA repair. These disorders are characterized by increased cancer risk, growth delays, and sensitivity to radiation, highlighting the importance of an intact cell cycle.
Conclusion
The cell cycle is a meticulously coordinated process essential for the growth, development, and maintenance of all living organisms. Its phases from the preparatory interphase to the dramatic events of mitosis and cytokinesis work in concert to ensure accurate DNA replication and cell division.
Regulated by an intricate network of cyclins, CDKs, and inhibitors, the cell cycle incorporates multiple checkpoints to prevent errors and maintain genetic integrity. When these regulatory systems fail, abnormalities such as uncontrolled division, mutation accumulation, and aneuploidy can lead to severe consequences, including cancer.
Advances in understanding cell cycle regulation have revolutionized medical fields like oncology, regenerative medicine, and gene therapy. By targeting specific phases or regulators of the cell cycle, modern treatments aim to selectively eliminate abnormal cells while preserving healthy tissues. Continued research into the molecular mechanisms governing the cell cycle promises to unlock new therapeutic possibilities and deepen our comprehension of life’s fundamental processes.
Frequently Asked Questions (FAQ)
What is the main purpose of the cell cycle?
The cell cycle allows cells to grow, duplicate their genetic material, and divide into two identical daughter cells. This process is essential for growth, tissue repair, and replacing old or damaged cells in living organisms.
What are the different phases of the cell cycle?
The cell cycle is divided into Interphase (which includes G₁ phase for cell growth, S phase for DNA replication, and G₂ phase for preparation) and the M phase, where mitosis and cytokinesis occur, resulting in two identical daughter cells.
Why are checkpoints important during the cell cycle?
Checkpoints monitor the cell’s progress at specific stages, ensuring that DNA is correctly replicated and any damage is repaired. They prevent the division of defective cells, thereby maintaining genetic stability and protecting against conditions like cancer.