Chromatography is a flexible analytical method in chemistry that is used to separate and examine mixtures of chemicals into their constituent parts. Chromatography comes in different forms, each with unique uses, procedures, and guiding principles. Below are 14 different types of chromatography and a brief explanation of its purposes, procedures, and guiding principles:
Table of Contents
Column Chromatography
Column Chromatography is a combination is passed through a column filled with a stationary phase (such as silica gel) and eluted with a mobile phase (liquid solvent) in column chromatography, a separation process. Different interactions between the mixture’s constituents and the stationary phase cause the components to separate according to attributes like size, charge, and polarity.
The principle of separation is the differential partitioning of a stationary phase (solid or liquid) from a mobile phase (liquid or gas).
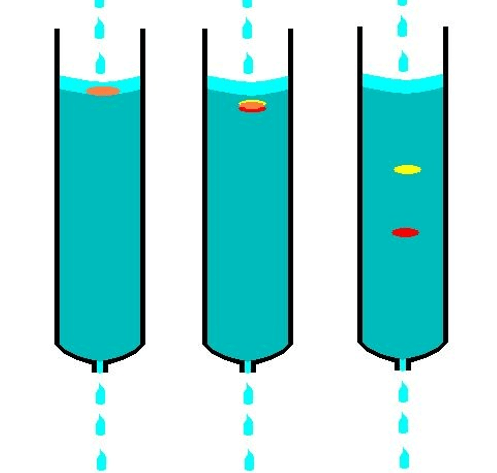
Procedure: The sample is poured over the top of a stationary phase-filled column and eluted with a mobile phase.
Applications: organic chemical purification; mixture separation
Affinity Chromatography
Affinity chromatography is a method of separation that uses the selective binding of particular biomolecules to a stationary phase that contains immobilised ligands to separate and purify those biomolecules.
The principle of affinity chromatography is to separate components according to how well they bind to the stationary phase.
Steps: entails eluting the target molecule once it has been bound to a ligand on the stationary phase.
Applications: Enzymes, proteins, and antibodies are purified.
Examples include separating particular proteins from a mixture
Anion Exchange Chromatography
Anion exchange chromatography is a method that uses interactions between charged molecules—like proteins or nucleic acids—and a stationary phase that possesses positively charged groups to separate and purify the molecules.
Principle of anion exchange chromatography: based on charge differences, separates
Steps: Anions bind to the positively charged stationary phase; ionic strength or pH changes cause elution.
Applications: DNA separation, protein purification.
As an example, sort DNA fragments based on size
Cation Exchange Chromatography:
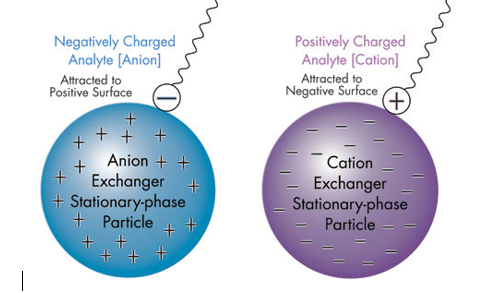
Cation exchange chromatography is a form of ion exchange chromatography (IEX), which is used to separate molecules based on their net surface charge. Cation exchange chromatography, more specifically, uses a negatively charged ion exchange resin with an affinity for molecules having net positive surface charges.
Principle: Charge differences (the reverse of anion exchange) are used to separate materials.
Steps: By changing the conditions, cations attach to the negatively charged stationary phase and elute.
Applications: Enzyme isolation and protein purification.
Examples include the charge-based separation of proteins.
Flash Chromatography
Flash Chromatography is a quick purification method involves eluting a mixture at high flow rates with a solvent after it has been passed through a column that has been loaded with a stationary phase.
Principle: Pre-packed columns are used in rapid column chromatography.
Process: Quicker than with column chromatography.
Uses: Fast cleansing.
Organic compound separation is one example.
Gas Chromatography
Gas chromatography is a stationary phase inside a column and a mobile phase of an inert gas, usually nitrogen or helium, provide the basis for the separation and analysis of volatile substances in a combination using gas chromatography (GC).
Based on how volatile substances vaporize and interact with a stationary phase, a principle is employed to separate them.
Procedure: The sample was vaporised and then run through a filter.
Applications: Compound analysis, volatile organic.
Gel Filtration Chromatography
Size exclusion chromatography, another name for gel filtration chromatography, is a method of separation that divides molecules according to size. Larger molecules move through the column more quickly and elute earlier, while smaller molecules are excluded from the pores and elute later. This is achieved by using a porous gel matrix in a column.
Theorem: Size-based separation; bigger molecules elute more quickly.
Steps: Sample travels through gel beads with pores in it.
Applications: molecular weight determination, protein purification.
Protein size separation is one example.
High-Performance Liquid Chromatography
HPLC, or high-performance liquid chromatography, is a potent analytical method that may be used to separate, identify, and measure different components in a mixture. A column filled with a stationary phase is pumped under high pressure with a liquid solvent containing the sample.
Principle: Liquid flows through a column under high pressure.
Procedure: Inject, segregate, and detect the sample.
Applications: drug testing, quantitative analysis.
Pharmaceutical analysis is one example.
Hydrophobic Interaction Chromatography (HIC):
A method of separation called hydrophobic interaction chromatography (HIC) is used to separate biomolecules according to how hydrophobic they are. Nonpolar portions of biomolecules can interact with the stationary phase through the use of a stationary phase containing hydrophobic ligands.
Principle: Hydrophobic interactions cause separation.
The sample binds to the stationary phase that is hydrophobic.
Applications: Protein extraction.
As an example: Separating proteins that are hydrophobic
Ion Exchange Chromatography:
Ion exchange chromatography is based on how charged molecules in a mixture interact with charged groups on a stationary phase, ion exchange chromatography is a technique used to separate and purify the molecules.
Principle: Based on ion charge, separates.
Steps: Ions attach to a stationary phase that is oppositely charged.
Applications: Metal ion separation, protein purification.
Amino acid separation is one example.
Paper Chromatography
Paper chromatography is a cheap and easy method for separating mixtures of chemicals is paper chromatography, which involves the use of a piece of paper or another cellulose-based material to allow the compounds to migrate differently from one another.
Theorem: Utilizes differential migration on filter paper to facilitate separation.
Steps: Solvent rises through capillary action when the sample is spotted on paper.
Applications: Examination of pigments, amino acids, and tiny compounds.
Plant pigment separation is one example.
Planar Chromatography
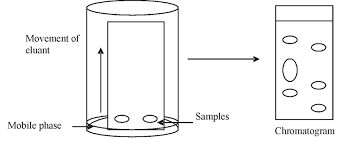
In planar chromatography, the stationary phase is not packed into a column but is instead immobilised on a flat surface, like a plate or sheet.
Concept: Based on paper chromatography, but with a solid stationary phase (alumina or silica gel, for example).
Steps: The stationary phase is filled with the solvent, and the sample is spotted on the plate.
Applications: Mixture analysis of chemicals.
One example is TLC, or thin-layer chromatography.
Reverse-Phase Chromatography (RPC)
Reverse-phase chromatography uses a polar mobile phase and a nonpolar stationary phase.
Principle: Polar mobile phase, hydrophobic stationary phase.
Steps: Load sample onto column; elute with organic solvent gradient.
Applications: Nucleic acid, peptide, and protein separation.
Analysing protein combinations is one example.
Supercritical Fluid Chromatography (SFC)
Supercritical fluids are used as the mobile phase in the separation process known as supercritical fluid chromatography (SFC).
Principle: Uses the mobile phase to be supercritical fluids, such as carbon dioxide.
Procedure: The sample is put into the supercritical fluid, and it separates.
Applications: Chiral chemical, lipid, and natural product analysis.
Enantiomer separation is one example.
Frequently Asked Questions(FAQ) of 14 types of chromatography
What is Chromatography?
Chromatography is a flexible analytical method in chemistry that is used to separate and examine mixtures of chemicals into their constituent parts. Chromatography comes in different forms, each with unique uses, procedures, and guiding principles.
What is Paper Chromatography?
Paper chromatography is a cheap and easy method for separating mixtures of chemicals is paper chromatography, which involves the use of a piece of paper or another cellulose-based material to allow the compounds to migrate differently from one another.
List any 3 types of Chromatography?
Among the 14 Chromatography the 5 chromatography types are
1.Column Chromatography
2.Gas Chromatography
3. Supercritical Fluid Chromatography (SFC)
Related Articles