Polymerase Chain Reaction is a technique used in molecular biology to create several copies of a certain DNA segment. The PCR technique, created in 1983 by Kary Mullis, facilitates the study and analysis of genetic material by enabling researchers to produce millions of copies of a certain DNA sequence from a tiny original sample.
Table of Contents
Types of PCR with Definition, Principle and Uses

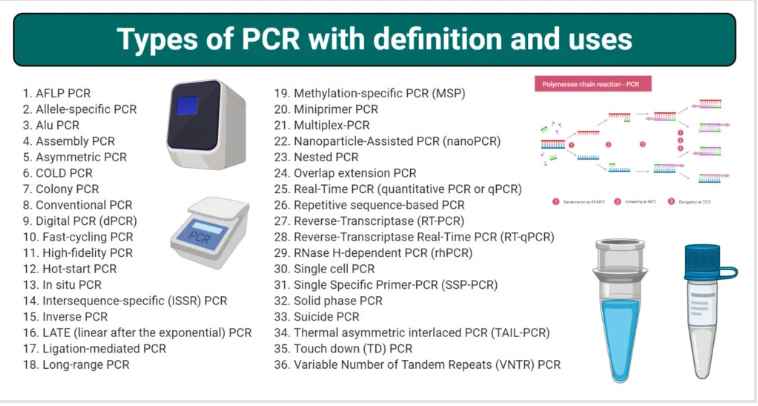
The 37 Types of PCR are explained below:
1. Amplified fragment length polymorphism (AFLP) PCR
AFLP is a molecular marker technique that finds polymorphisms in DNA sequences by combining the concepts of PCR and restriction enzyme digestion.
Principle:
- Digestion: Restrictions enzymes are used to break down genomic DNA.
- Ligation: Restrictions fragments’ sticky ends are ligated to adapters.
Uses:
- Genetic Fingerprinting: Creates a distinct pattern of DNA fragments that looks like a fingerprint.
- Plant Breeding: Used in plant breeding to facilitate marker-assisted selection.
2. Allele-specific PCR
The Amplification Refractory Mutation System (ARMS-PCR), commonly known as allele-specific PCR (AS-PCR), is a powerful method for identifying particular alleles within a gene.
Principles:
- Primer Design: AS-PCR depends on the creation of primers that amplify a single allele only.
- Detection: A mutation results in the production of two distinct alleles for a gene: a mutant and a normal one.
Uses:
- Genotyping: Allows genetic variations within populations or individuals to be analyzed.
- Diagnostics: Aids in researching the correlation between particular alleles and characteristics or diseases.
3. ALU PCR
ALU PCR is a method that amplifies particular Alu elements particular Alu elements.
Principles:
- Primer Design: Alu sequences’ conserved regions serve as the binding site for primers.
- Amplification: During PCR, these primers anneal to Alu elements dispersed throughout the genome.
Uses:
- Retrotransposon Insertion Detection: To determine whether Alu retrotransposon insertions exist in genomic DNA, Alu PCR is utilized.
- Amplification of Alu Elements: This process amplifies particular genome regions that have repetitive DNA sequences called Alu elements.
4. Assembly PCR
One method for combining two gene-sized fragments of DNA into one is assembly PCR.
Principles:
- Primer Design: Creating primers that overlap with adjacent pieces for every fragment.
- PCR Amplification: Using PCR to independently amplify each fragment.
Uses:
- Cloning: Allows recombinant DNA molecules to be produced, which is useful for genetic engineering and gene cloning.
- Drug Development: DNA sequences are engineered for protein expression, drug target validation.
5. Asymmetric PCR
Asymmetric PCR is a variant of the conventional PCR method that is intended to amplify one strand of the target DNA more than the other.
Principles:
- Single-Stranded DNA Production: An excessive amount of a primer specific to one strand is used.
- Primer Design: A ten-fold higher volume of the target-specific primer is used.
Uses:
- Selective Strand Amplification: One DNA strand is amplified more favorably than the other by asymmetric PCR.
- Unidirectional Amplification: Generates more single-stranded DNA than double-stranded DNA in terms of yield.
6. COLD PCR
A modified polymerase chain reaction (PCR) technique called COLD-PCR (co-amplification at lower denaturation temperature PCR) is used to extract variant alleles from a combination of DNA carrying mutations and wildtype DNA.
Principles:
- Critical Temperature: Employs a critical denaturation temperature (Tc) that is less than the PCR denaturation temperature standard.
- High Sensitivity: Improves the PCR product’s ability to identify low-abundance mutations.
Uses:
- Increased Sensitivity: Compared to conventional PCR techniques, it provides increased sensitivity.
- Extremely Effective: Offers excellent mutation enrichment effectiveness while preserving amplification specificity.
7.Colony PCR
Colony PCR is a technique used in molecular cloning protocols to rapidly examine bacterial colonies produced following the transformation process.
Principles:
- Colony Picking: Take a tiny sample of an agar plate’s bacterial colony.
- PCR Reaction: Combine this lysate straight to the buffer, primers, dNTPs, and Taq polymerase mixture for the PCR reaction.
Uses:
- Time-Efficient: Compared to conventional methods, it offers a rapid and labor-saving technique.
- Recombinant Clone Identification: Assists in locating recombinant clones that have plasmids with inserted DNA fragments.
8. Conventional PCR
The commonly used method of “molecular photocopying,” also known as conventional Polymerase Chain Reaction (PCR), amplifies minute quantities of DNA and RNA from a sample.
Principles:
- Denaturation: To differentiate double strands in DNA, heat it.
- Extension: To create new DNA strands, DNA polymerase adds nucleotides to primers.
Uses:
- Food Safety Testing: Used in food safety testing to find foodborne pathogens in food products, such as E. Coli, Salmonella, or Listeria.
- Agricultural Research: Used in agricultural research to find genetically modified organisms (GMOs) in crops.
9. Digital PCR (dPCR)
A sensitive and accurate molecular method for measuring and detecting nucleic acids is called digital PCR (dPCR).
Principles:
- Partitioning: Thousands of distinct reactions are created from the sample, each containing one or no target molecules.
- Amplification: Each reaction involves PCR, and amplification happens on its own.
Uses:
- Absolute Quantification: By counting individual DNA molecules, this method enables accurate analysis of copy number variations.
- Single-Molecule Resolution: Provides single-molecule resolution, enabling accurate single-cell nucleic acid molecule analysis.
10. Fast-cycling PCR
A variation on traditional PCR known as “fast-cycling PCR” uses optimized reaction conditions to shorten the overall amplification time without sacrificing efficiency or specificity.
Principles:
- Optimal Reaction Conditions: Makes use of adjusted cycling parameters, including shortened denaturation, in addition to higher temps.
- Improved Enzymes: To support faster cycling conditions, thermostable DNA polymerases with higher processivity and fidelity are used.
Uses:
- Accelerated PCR Process: By using fast-cycling PCR, PCR reactions can be amplified more quickly.
- Quick Results: Compared to standard PCR, this method allows for quicker completion.
11. High-fidelity PCR
In order to achieve high accuracy in DNA amplification, high-fidelity PCR (Polymerase Chain Reaction) uses DNA polymerases with proofreading capabilities.
Principles:
- Extension: Nucleotides are added to the primers by high-fidelity DNA polymerase.
- Proofreading: To ensure high accuracy, mismatched nucleotides are corrected by the polymerase’s 3′ to 5′ exonuclease activity.
Uses:
- Structural biology: Offers accurate DNA sequences for the structural analysis of nucleic acids and the investigation of protein-DNA interactions.
- Genetic Engineering: Essential for applications like synthetic biology and CRISPR-based gene editing that need precise DNA sequences.
12. High-Resolution Melt (HRM) PCR
HRM PCR is a post-PCR analysis technique that uses differences in double-stranded DNA melting behavior to find genetic variants, mutations, and polymorphisms in DNA.
Principles:
- Amplification: PCR is used to amp up DNA.
- Melting: A steady heat is applied to the amplified DNA.
Uses:
- Epigenetic Studies: Examines DNA methylation trends.
- Pathogen Detection: Identifies different types of microorganisms.
13. Hot-start PCR
A variant of the conventional PCR method called “hot-start PCR” works by only activating the DNA polymerase at elevated temperatures, thus reducing primer-dimer formation and non-specific amplification.
Principles:
- Activation by Heat: The DNA polymerase is first activated by a high temperature step.
- Standard PCR Cycling: Less non-specific amplification occurs during the PCR’s denaturation, annealing, and extension stages.
Uses:
- Enhanced Sensitivity: Reduces background amplification to enable the identification of low-abundance targets.
- Increased Yield: Produces greater yields of the targeted good.
14. In situ PCR
The method known as “in situ PCR” allows genetic material to be localized within the histological context of fixed cells or tissue sections by directly amplifying DNA or RNA sequences within them.
Principles:
- Amplification: The sample is directly treated with PCR reagents, and thermal cycling is carried out.
- Detection: Labeled probes or labeled nucleotides are used in situ to identify amplified DNA or RNA.
Uses:
- Research on Cancer: Determines the distribution of genetic mutations in cancerous tissues.
- Developmental Biology: Examines how genes express themselves both spatially and temporally during development.
15. Intersequence-specific (ISSR) PCR
A molecular method called isozyme-sequence repeat PCR (ISSR PCR) is used to create polymorphic DNA markers for genetic analysis by amplifying regions between microsatellites or simple sequence repeats (SSRs).
Principles:
- Amplification: The DNA segments between two neighboring SSRs are amplified by the primer.
- Polymorphism Detection: Genetic diversity is reflected in variations in the length and sequence of amplified regions.
Uses:
- Breeding programs: help choose desirable and genetically varied features in plants and animals.
- Conservation Genetics: By examining genetic variability, conservation genetics aids in the management and preservation of endangered species.
16. Inverse PCR
A variant of the PCR process known as “inverse PCR” uses primers orientated in the opposite way to amplify DNA sequences surrounding a known sequence area.
Principles:
- Limitation Digestion: To produce fragments, restriction enzymes are used to break down the DNA.
- Circularization: To create circular DNA, the digested DNA fragments are ligated.
Uses:
- Genomic Mapping: This method maps the genomic areas’ structures around recognized genes or markers.
- Molecular Characterization: Defines the locations where transgenes or viral genomes integrate into host DNA.
17. LATE (Linear After the Exponential) PCR
LATE PCR is a modification of standard PCR designed to generate single-stranded DNA products by employing unequal primer concentrations, resulting in a linear amplification phase after the initial exponential phase.
Principles:
- Asymmetric priming: Employs different ratios of forward and reverse primer concentrations.
- Exponential Phase: First cycles, like those of a typical PCR, amplify the target DNA exponentially.
Uses:
- Probe-Based Detection: Because single-stranded DNA predominates, this technique makes it easier to use probes in diagnostic experiments.
- Studies on Gene Expression: Beneficial for experiments that use single-stranded templates to investigate gene expression.
18. Ligation-mediated PCR
A molecular biology method called ligation-mediated PCR (LM-PCR) amplifies DNA fragments with specified ends by combining ligation and PCR.
Principles:
- Adaptors are used to ligate DNA segments with suitable cohesive ends.
- Primer-binding sites are present in adaptors.
Uses:
- Genomic walking: The process of identifying unidentified DNA sequences next to recognized ones.
- Amplification of Particular Segments: DNA fragments with known end sequences are amplified.
19. Long-range PCR
DNA fragments longer than the conventional PCR limit (often greater than 5 kb) can be amplified using a technique called long-range PCR (Polymerase Chain Reaction).
Principles:
- Steps for denaturation, annealing, and extension (as in traditional PCR).
- Large DNA fragments (up to 30 kb or more) are amplified.
Uses:
- Amplification of Massive Genomic Regions: Enables the amplification of big plasmids, genomic regions, or whole genes.
- Genomic Sequencing: This method amplifies lengthy DNA segments with high fidelity in order to prepare DNA samples for sequencing.
20. Methylation-specific PCR (MSP)
A method for identifying DNA methylation, a significant epigenetic alteration, at certain gene loci is called methylation-specific PCR, or MSP.
Principles:
- PCR Amplification: Two primer sets—one particular to methylated DNA and the other to unmethylated DNA—are utilized.
- Differentiation: The methylation state of the DNA at particular locations is shown by the presence or lack of PCR results.
Uses:
- Cancer research: identifying abnormal DNA methylation patterns connected to cancers.
- Epigenetic Studies: Examining mechanisms of regulation and gene silencing.
21. Miniprimer PCR
Miniprimer PCR is a PCR variation that amplifies DNA using extremely short primers, usually 10–15 nucleotides long.
Principles:
- Short Primers: Shorter than conventional PCR primers, miniprimers are used.
- Annealing: The likelihood of locating appropriate binding sites is increased by the ability of short primers.
Uses:
- Ancient and Forensic DNA Analysis: Useful for amplifying damaged DNA in situations where longer primers might not bind well.
- Environmental DNA Studies: Using short sequences that have been conserved, microbial or environmental DNA can be amplified.
22. Multiplex-PCR
Multiplex PCR is a technique that uses multiple sets of primers to allow the simultaneous amplification of numerous target DNA sequences in a single PCR reaction.
Principles:
- Simultaneous Amplification: In the same reaction tube, all target sequences are amplified simultaneously under the same circumstances.
- Optimization: To guarantee effective and targeted amplification of all targets, meticulous adjustment of primer concentrations and PCR conditions is necessary.
Uses:
- Genetic testing: Finding several genetic markers or mutations at once.
- Identification of Pathogens: Identifying several pathogens in a single test.
23. Nanoparticle-Assisted PCR (nanoPCR)
A version of the conventional PCR method known as “nanoparticle-Assisted PCR” (nanoPCR) uses nanoparticles to improve the yield, specificity, and efficiency of DNA amplification.
Principles:
- Nanoparticles: The PCR reaction is supplemented with metallic or non-metallic nanoparticles, such as silica, gold, or silver.
- Enhanced Performance: Nanoparticles stabilize DNA polymerase, reduce non-specific binding, and enhance heat transfer.
Uses:
- Greater Sensitivity: Compared to traditional PCR, this method is more sensitive.
- Diagnostic Applications: Improving pathogen, genetic disease, or cancer marker detection.
24. Nested PCR
A method called nested PCR amplifies DNA using two sets of primers in two consecutive rounds to improve sensitivity and specificity.
Principles:
- First PCR: Using outside primers, a target sequence is amplified.
- Second PCR: Nested inner primers within the first amplicon, using a piece of the first PCR result as a template.
Uses:
- Low-Abundance DNA Detection: Makes unusual DNA sequences easier to find.
- Pathogen Identification: Increases the precision of pathogen identification.
25. Overlap extension PCR
Expansion of overlap By joining many PCR fragments, the PCR process is used to construct DNA sequences with specified insertions, deletions, or changes.
Principles:
- Fragment Amplification: PCR is used to independently amplify many DNA fragments with overlapping ends.
- Overlap Region: The pieces can anneal to one another when they have overlapping regions on their ends.
Uses:
- Site-directed mutagenesis: The process of adding certain mutations to a DNA sequence.
- Gene fusion and cloning: Joining genes to form chimeric genes.
26. Real-Time PCR (quantitative PCR or qPCR)
Real-time PCR, sometimes referred to as quantitative PCR or qPCR, is one of the most important types of PCR which is a sensitive molecular biology method that measures and amplifies DNA sequences in real time while the PCR reaction is underway.
Principles:
- Fluorescent Probes: When attached to the PCR product, fluorescent probes that release fluorescence are used.
- Cycle Monitoring: Real-time monitoring of DNA amplification is made possible by measuring fluorescence following each PCR cycle.
Uses:
- Gene Expression Analysis: Measuring mRNA levels to examine trends in gene expression.
- Pathogen detection: The identification and measurement of microbiological pathogens in medical specimens.
27. Repetitive sequence-based PCR
As a molecular biology approach, repetitive sequence-based PCR (rep-PCR) amplifies DNA areas with significant sequence variability by using primers that target repeating sequences.
Principles:
- Repeated Sequences: Primers made to attach to DNA repeats found all over the genome are used.
- Amplification: By amplifying DNA sections bordered by repeating sequences, PCR creates distinct patterns resembling fingerprints.
Uses:
- Strain Typing: Distinguishing between bacterial or other organism isolates or strains.
- Epidemiological Studies: Monitoring the transmission of contagious illnesses and locating the origins of epidemics.
28. Reverse-Transcriptase (RT-PCR)
Reverse transcription polymerase chain reaction, or RT-PCR, is also one of the types of PCR that amplifies and detects RNA molecules by first utilizing the reverse transcriptase enzyme to convert them into complementary DNA (cDNA).
Principles:
- Reverse Transcription: A primer and the reverse transcriptase enzyme are used to reverse transcribe RNA into cDNA.
- PCR Amplification: Next, using gene-specific primers, the cDNA is amplified by PCR.
Uses:
- Gene Expression Analysis: Measuring mRNA concentrations to examine patterns of gene expression.
- Viral Load Quantification: Diagnosing and tracking viral infections by measuring the amounts of viral RNA in clinical samples.
29. Reverse-Transcriptase Real-Time PCR (RT-qPCR)
Transcription in Reverse Real-Time PCR (RT-qPCR) is a molecular biology technique that uses real-time PCR along with reverse transcription of RNA into cDNA to quantify the levels of gene expression.
Principles:
- Reverse Transcription: The reverse transcriptase enzyme is used to reverse transcribe RNA into complementary DNA (cDNA).
- Real-Time PCR: Next, using fluorescent probes or dyes, the cDNA is amplified quantitatively in real-time using PCR.
Uses:
- Biomarker detection: The process of locating and measuring particular RNA biomarkers linked to illnesses.
- Drug Efficacy Testing: Measuring how medications or therapies affect the levels of gene expression.
30. RNase H-dependent PCR (rhPCR)
RNA in RNA-DNA hybrid molecules is selectively degraded by RNase H in a PCR process known as RNase H-dependent PCR (rhPCR).
Principles:
- RNA-DNA Hybrids: For PCR amplification, RNA templates are hybridized to DNA primers.
- RNase H Activity: In RNA-DNA hybrids, the RNase H enzyme specifically breaks down the RNA strand.
Uses:
- RNA Detection: Does not require reverse transcription and enables targeted amplification of RNA sequences.
- RNA Mutation Analysis: Enables RNA molecules to be modified or mutated more easily.
31. Single cell PCR
An analysis and amplification of DNA or RNA from individual cells can be achieved by the molecular biology technique known as single cell PCR.
Principles:
- Cell Isolation: Methods such as microfluidic or micromanipulation are frequently used to extract individual cells.
- Cell lysis: To liberate their nucleic acids, cells undergo cell lysis (DNA or RNA).
Uses:
- Gene Expression Profiling: To comprehend cellular heterogeneity, single-cell analysis of gene expression patterns is performed.
- Cancer Research: Using single-cell analysis, research on tumor heterogeneity and clonal evolution is conducted.
32. Single Specific Primer-PCR (SSP-PCR)
SSP-PCR, or single-specific primer amplification, is a PCR method that amplifies particular DNA sequences with just one primer.
Principles:
- Primers Annealing: The primer attaches itself to the target DNA’s complementary sequence.
- DNA Amplification: A particular product is produced by PCR, which amplifies the DNA area that the primer surrounds.
Uses:
- Pathogen detection: The process of identifying particular microbial species or pathogens using their distinct DNA sequences.
- Molecular Haplotyping: Utilizing particular DNA markers to ascertain an individual’s genetic haplotype.
33. Solid phase PCR
Using a solid support to immobilize one of the PCR components, solid phase PCR allows for easier PCR product separation and purification.
Principles:
- Immobilization: A single PCR component, like primers or templates, is affixed to a stable support, such as a microarray or magnetic beads.
- PCR Amplification: The appropriate free component and the immobilized component are mixed together and used in PCR.
Uses:
- High-Throughput Screening: Enabling the analysis and processing of several PCR reactions in simultaneously.
- Nucleic Acid Purification: Facilitating the separation of complicated sample PCR products.
34. Suicide PCR
A PCR technique known as “suicide PCR” makes use of modified primers to stop amplification after a certain number of PCR cycles.
Principles:
- Modified Primers: In order to avoid their binding in next PCR cycles, primers have undergone a chemical modification or deletion.
- Restricted Amplification: After a set number of cycles, PCR amplification continues normally until the modified primers stop it.
Uses:
- Limiting Amplification: Effective in preventing non-specific amplification and managing the amplification of particular targets.
- Library Construction: By restricting PCR amplification to specific locations, you can help build DNA libraries.
35. Thermal asymmetric interlaced PCR (TAIL-PCR)
TAIL-PCR, or thermal asymmetric interlaced PCR, is a PCR method that uses primer annealing temperature differences to amplify DNA sequences next to known areas.
Principles:
- Annealing Temperature Gradient: A sequence of nested primers, each with a distinct annealing temperature, are used in PCR.
- Stepwise Amplification: First cycles enhance sections proximal to the known sequence preferentially using high annealing temperatures.
Uses:
- Gene mapping: Assists in mapping unknown sequences next to sections of known DNA.
- Identification of Regulatory Elements: Facilitates the determination of enhancers, promoters, and other DNA regulatory elements.
36. Touch down (TD) PCR
During the first few cycles of Touch-Down (TD) PCR, the annealing temperature is progressively lowered in an effort to increase specificity and decrease nonspecific amplification.
Principles:
- Gradual Temperature Decrease: In consecutive cycles, the annealing temperature is gradually lowered.
- Optimization: The process seeks to minimize nonspecific amplification while determining the ideal annealing temperature for selective amplification.
Uses:
- Enhanced Specificity: Lessens nonspecific amplification to increase specificity.
- Complicated Templates: Good for boosting secondary structures or complex templates with a high GC content.
37. Variable Number of Tandem Repeats (VNTR) PCR
A genetic variant known as Variable Number of Tandem Repeats (VNTR) is typified by the presence of different numbers of short DNA sequences that repeat in tandem.
Principles:
- DNA Extraction: A sample’s DNA is taken out.
- PCR Amplification: The VNTR region is amplified using a Polymerase Chain Reaction (PCR) using primers specific to the flanking regions of the VNTR.
Uses:
- Paternity testing: VNTRs can be used to determine biological relationships, such as paternity.
- Population Genetics: VNTRs are helpful in the study of genetic diversity and population structure.
Summary: Here, we explained about the 37 types of PCR along with its principles and uses. There are over 37 types of PCR, each tailored for specific applications. Each types of PCR is defined by its unique modifications. These types of PCR are widely used in genetic research, clinical diagnostics, forensic science, medical applications.
Frequently Asked Questions(FAQ)
What is PCR?
A molecular biology method for amplifying particular DNA sequences is called PCR (Polymerase Chain Reaction).
How many types of PCR are there?
There are basically 37 types of PCR. They are: Standard PCR, Real-Time PCR (qPCR), Reverse Transcription PCR (RT-PCR), Multiplex PCR, Touchdown PCR, Nested PCR, Hot Start PCR, Digital PCR (dPCR), Allele-Specific PCR,
Long PCR, High-Fidelity PCR, In Situ PCR, Quantitative Reverse Transcription PCR (qRT-PCR), Inverse PCR, Asymmetric PCR, Overlap Extension PCR, Colony PCR, Methylation-Specific PCR (MSP), Assembly PCR, Anchored PCR, AP-PCR (Arbitrary Primed PCR), COLD-PCR (Co-amplification at Lower Denaturation temperature PCR), DD-PCR (Differential Display PCR), EMSA-PCR (Electrophoretic Mobility Shift Assay PCR), Fast PCR, Fusion PCR, Genomic PCR, Intersequence-Specific PCR (ISSR-PCR), Ligation-Mediated PCR (LM-PCR), Mini-PCR, Nanoparticle PCR, Overlap Extension PCR, Quantitative Competitive PCR (QC-PCR), RAPD-PCR (Random Amplified, Polymorphic DNA PCR), Recombinant PCR, Solid Phase PCR ,Suicide PCR, Tailed PCR, Universal Fast Walking (UFW-PCR).
Related Articles