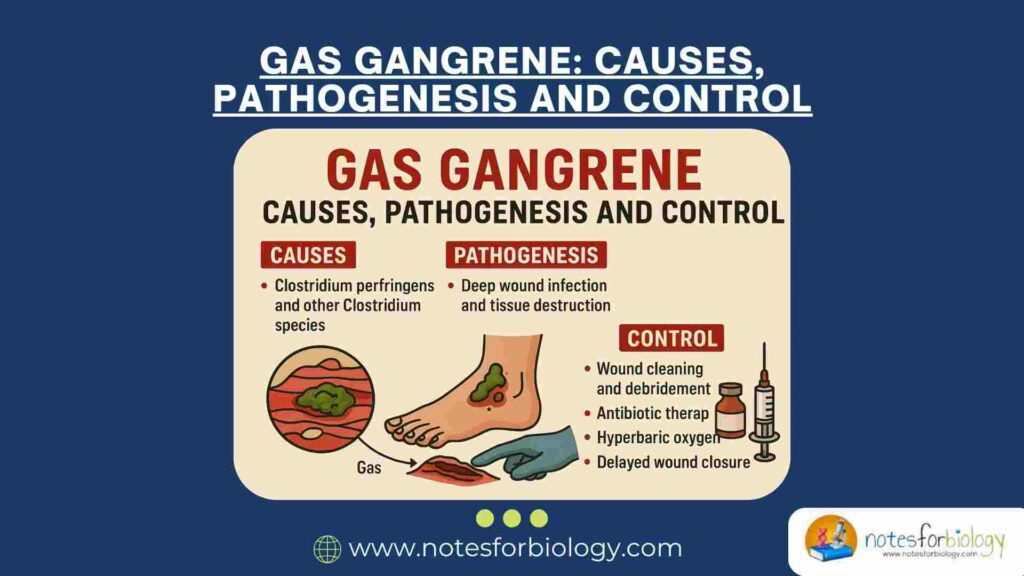
Gas gangrene, also known as clostridial myonecrosis, is a rapidly progressive and life-threatening infection characterized by extensive muscle necrosis, gas production, and systemic toxicity. The disease commonly occurs in deep wounds contaminated with soil or fecal matter and is often associated with traumatic injuries, surgical procedures, or compromised blood supply. Gas gangrene demands immediate medical attention, as delayed treatment significantly increases morbidity and mortality.
Summary of Gas Gangrene
- Gas gangrene is a deadly infection caused by Clostridium bacteria producing toxins and gas in wounds.
- It causes severe pain, tissue death, and gas buildup; diagnosed by symptoms and lab tests.
- Treatment requires urgent surgery and antibiotics; prevention involves good wound care.
Table of Contents
Causative Agents of Gas Gangrene
Gas gangrene is primarily caused by bacteria belonging to the genus Clostridium, which are anaerobic, gram-positive, spore-forming bacilli. These organisms are ubiquitous in soil, sewage, and the gastrointestinal tracts of humans and animals. They become pathogenic when introduced into necrotic or oxygen-deprived tissues, typically through contaminated wounds or surgical incisions.
Clostridium perfringens
Clostridium perfringens is responsible for the majority of gas gangrene cases, accounting for 80–90% of infections. It is a gram-positive, non-motile, spore-forming bacillus capable of producing a variety of toxins. Type A strains are most commonly implicated in human infections, producing alpha toxin, a lethal phospholipase that plays a central role in tissue destruction. The bacterium exists widely in soil, dust, and the intestinal tract, surviving harsh environments due to its spore-forming ability.
Clostridium novyi
This species is a motile, spore-forming, anaerobic bacillus that, although less common than C. perfringens, can cause highly virulent infections. Clostridium novyi produces multiple exotoxins and enzymes that contribute to rapid tissue necrosis. Type A strains are associated with human disease, typically arising in wounds with impaired blood flow where anaerobic conditions favor bacterial proliferation.
Clostridium septicum
Clostridium septicum is particularly notable for causing spontaneous, non-traumatic gas gangrene, especially in patients with underlying malignancies. It is motile, spore-forming, and capable of infecting well-oxygenated tissues. Its exotoxins rapidly destroy tissues, and its association with gastrointestinal and hematological cancers makes its isolation in clinical settings a potential indicator of hidden malignancy.
Clostridium histolyticum
Though less frequently isolated, Clostridium histolyticum contributes to polymicrobial gas gangrene. It produces collagenase and hyaluronidase, enzymes that break down connective tissue, promoting rapid bacterial spread through muscle and subcutaneous planes. This species is commonly found in soil and animal feces.
Clostridium sordellii
Clostridium sordellii is a rare cause of gas gangrene but is highly lethal when infection occurs. It produces powerful toxins that lead to capillary leakage, tissue necrosis, and systemic toxicity. Cases have been linked to trauma-related wounds and obstetric procedures. The infection progresses rapidly and often results in a poor prognosis without prompt intervention.
Polymicrobial Infections
Occasionally, gas gangrene results from mixed infections involving anaerobic Clostridium species and aerobic organisms like Staphylococcus aureus or Escherichia coli. In these cases, the aerobes initially reduce tissue oxygen tension, creating a favorable anaerobic environment for clostridial proliferation. Though C. perfringens typically remains the primary pathogen, mixed infections complicate treatment and can worsen patient outcomes.
Virulence Factors of Gas Gangrene
The severity and rapid progression of gas gangrene are driven by several potent virulence factors produced by Clostridium species. These include powerful exotoxins and tissue-degrading enzymes that facilitate bacterial proliferation, tissue destruction, and systemic toxicity.
Alpha Toxin
Alpha toxin, produced predominantly by C. perfringens, is a phospholipase C (lecithinase) that hydrolyzes phospholipids in cell membranes. This activity leads to the lysis of red blood cells, leukocytes, muscle cells, and endothelial cells, causing tissue necrosis, hemolysis, and increased vascular permeability. Additionally, it contributes to cardiovascular instability by promoting platelet aggregation and disrupting normal blood flow.
Theta Toxin
Produced by C. perfringens, theta toxin is a pore-forming cytolysin that inserts into cell membranes, leading to cell lysis and necrosis. It damages vascular endothelium and induces leukocyte degeneration, impairing the immune response and enhancing bacterial survival within infected tissue.
Enzymes: Collagenase, Hyaluronidase, and DNase
Various enzymes aid in tissue degradation and bacterial spread. Collagenase breaks down collagen in connective tissue, facilitating bacterial migration. Hyaluronidase degrades hyaluronic acid in the extracellular matrix, while DNase reduces pus viscosity by breaking down DNA from lysed cells, promoting the diffusion of bacteria and toxins.
Gas Formation
Gas production within tissues is a distinctive feature of gas gangrene. Through anaerobic fermentation of carbohydrates, Clostridium species generate hydrogen and carbon dioxide. Gas accumulates in muscle compartments and subcutaneous tissues, creating crepitus and contributing to tissue separation, further reducing local oxygen supply and sustaining anaerobic conditions.
Pathogenesis
The pathogenesis of gas gangrene involves a rapid sequence of bacterial entry, multiplication, toxin production, tissue destruction, and systemic involvement. It typically follows the contamination of devitalized tissue with Clostridium spores, especially under anaerobic conditions.
Entry into Tissue
Infection commonly begins after trauma, surgery, or injuries introducing Clostridium into deep, poorly oxygenated tissues. Devitalized and necrotic tissues provide an ideal anaerobic environment for bacterial germination. Contaminated open wounds and compound fractures are typical portals of entry.
Bacterial Multiplication
Once in anaerobic conditions, Clostridium species multiply rapidly, consuming remaining oxygen and promoting an environment suitable for further bacterial growth. Their proliferation within necrotic tissue enables the production of powerful exotoxins and tissue-degrading enzymes.
Toxin Production and Tissue Destruction
Exotoxins, notably alpha and theta toxins, rapidly destroy cell membranes, muscle tissue, and blood vessels. Simultaneously, collagenase and hyaluronidase facilitate bacterial movement through tissue planes. This destruction results in tissue necrosis, hemorrhagic edema, and rapid disease progression.
Gas Formation and Local Effects
During metabolism, the bacteria produce hydrogen and carbon dioxide, accumulating within tissues and causing crepitus. Gas pressure compromises blood flow, leading to further tissue ischemia, necrosis, and bacterial proliferation, exacerbating local and systemic toxicity.
Systemic Toxicity
Toxins and inflammatory mediators enter the bloodstream, resulting in systemic symptoms like fever, hypotension, tachycardia, and confusion. Hemolysis contributes to anemia and renal damage. Without rapid intervention, patients may develop septicemia, shock, and multiorgan failure.
Clinical Features of Gas Gangrene
Gas gangrene presents with a combination of rapidly progressing local and systemic symptoms, typically emerging within 6 to 48 hours of injury.
Local Symptoms
The earliest and most characteristic symptom is severe, disproportionate pain at the infection site. The area becomes swollen, tense, and pale, soon developing a dusky discoloration. Crepitus is often palpable due to gas within tissues. The wound may discharge a thin, foul-smelling, serosanguinous fluid, and hemorrhagic bullae may form.
Systemic Symptoms
Systemic manifestations include high-grade fever, tachycardia, hypotension, and signs of systemic toxicity. Hemolysis causes pallor, anemia, and hemoglobinuria. In advanced cases, septic shock, renal failure, and multiorgan dysfunction occur, posing a significant risk of mortality.
Laboratory Diagnosis of Gas Gangrene
Prompt diagnosis of gas gangrene combines clinical evaluation with laboratory and imaging investigations.
Microscopic Examination
A Gram stain of wound discharge typically reveals gram-positive, large rectangular bacilli without spores. The absence of leukocytes is characteristic, as toxins destroy white blood cells early in infection.
Culture
Anaerobic culture of wound exudate or tissue samples on blood agar confirms Clostridium species. C. perfringens colonies exhibit large, spreading growth with a double zone of hemolysis on blood agar.
Imaging
Radiographs and CT scans detect gas pockets in soft tissues, providing a valuable diagnostic clue. The presence of gas bubbles in muscle compartments supports the clinical diagnosis of gas gangrene.
Hematological and Biochemical Tests
Blood tests often show anemia due to hemolysis, leukocytosis, metabolic acidosis, and elevated renal function markers. Blood cultures may detect Clostridium species in severe, systemic cases.
Treatment of Gas Gangrene
Gas gangrene requires immediate, aggressive treatment combining surgical, antimicrobial, and supportive therapies.
Surgical Management
Prompt and extensive surgical debridement is critical to remove all necrotic tissue and halt disease progression. In severe cases, amputation of affected limbs may be necessary. Multiple debridements may be required depending on infection severity.
Antibiotic Therapy
High-dose intravenous antibiotics should be initiated immediately. Penicillin is the drug of choice, often combined with clindamycin to inhibit toxin synthesis. Broad-spectrum coverage is used initially until culture results guide targeted therapy.
Supportive Care
Supportive management includes fluid resuscitation, blood transfusions for hemolysis, electrolyte correction, and monitoring for renal impairment. Dialysis may be required in cases of renal failure.
Hyperbaric Oxygen Therapy
Hyperbaric oxygen therapy (HBOT) delivers 100% oxygen under high pressure, inhibiting anaerobic bacteria and enhancing neutrophil function. It is an effective adjunct to surgical and medical treatments.
Immunotherapy
Experimental use of antitoxins, such as hyperimmune globulin or monoclonal antibodies against alpha toxin, has been considered in severe cases, though it is not widely adopted in clinical practice.
Pain and Wound Management
Analgesics are provided to control severe pain. Wound care includes daily dressing changes, antiseptic irrigation, and monitoring for signs of recurrent necrosis or secondary infections.
Prevention and Control of Gas Gangrene
Effective prevention of gas gangrene involves early wound care, aseptic surgical practices, and public health measures.
Wound Care
Prompt and thorough cleaning of contaminated wounds reduces infection risk. Necrotic tissue should be removed early, and open wounds irrigated with antiseptics. Closed injuries should be closely monitored.
Aseptic Techniques in Surgery
Maintaining strict asepsis during surgery, particularly gastrointestinal and trauma procedures, minimizes contamination risks. Prophylactic antibiotics may be administered in high-risk cases.
Public Health Measures
Public awareness of proper first aid and wound care, especially in disaster or military settings, reduces infection rates. Rapid evacuation and early treatment of injuries in these contexts are essential.
Surveillance and Early Detection
High clinical suspicion and early laboratory diagnosis are vital for effective control. Prompt recognition and intervention before systemic complications develop significantly improve survival rates.
Early Detection in High-Risk Patients
In patients with malignancies or immunosuppression, spontaneous gas gangrene due to C. septicum should be considered when unexplained tissue infections occur. High clinical suspicion leads to early treatment and improved outcomes.
Vaccination Research
Although no vaccine currently exists, research is ongoing into toxoid-based vaccines targeting alpha toxin and other virulence factors. These may offer protection to high-risk individuals in the future.
Conclusion
Gas gangrene remains one of the most feared soft tissue infections due to its rapid progression, severe tissue destruction, and high mortality rate. It is primarily caused by Clostridium perfringens and related species that thrive in devitalized, oxygen-poor tissues. The disease manifests with sudden, intense pain, tissue necrosis, and systemic toxicity. Prompt surgical debridement, combined with appropriate antibiotics and supportive therapy, forms the cornerstone of treatment. Preventive strategies such as proper wound care, aseptic techniques, and early clinical recognition are vital in reducing disease incidence. Advances in adjunctive therapies like hyperbaric oxygen and immunotherapy continue to enhance patient outcomes, but early diagnosis and aggressive intervention remain the most effective approach in managing gas gangrene.
Frequently Asked Questions (FAQ)
What is the difference between gangrene and gas gangrene?
Gangrene is tissue death due to loss of blood supply, while gas gangrene is a severe form caused by bacterial infection that produces gas in the tissues.
What is the cause of gas gangrene?
Gas gangrene is caused mainly by the bacterium Clostridium perfringens.
What is another name for gas gangrene?
Gas gangrene is also called clostridial myonecrosis.