Chemical reactions are essential processes that drive the transformations of matter around us. From biological systems to industrial production, countless changes occurring in the natural and artificial world depend on these chemical processes. By studying chemical reactions, we gain insight into how substances interact, break down, or combine to form entirely new materials.
In this article, we will explore the fundamental nature of chemical reactions, discuss their types, characteristics, and applications, and examine the factors that influence how fast and efficiently they occur. Special attention will be given to balancing chemical equations, understanding catalysts and enzymes, and recognizing real-life applications of these concepts.
Summary of Chemical Reactions
- Chemical reactions involve the transformation of reactants into products through the breaking and forming of chemical bonds, following the law of conservation of mass.
- They are classified into several types, including synthesis, decomposition, single replacement, double replacement, combustion, and redox reactions, each with distinct characteristics and applications.
- Reaction rates are influenced by factors like temperature, concentration, surface area, catalysts, and pressure, and can be measured using changes in mass, volume, color, or conductivity.
Table of Contents
Importance of Chemical Reactions in Science and Daily Life
Chemical reactions play an indispensable role in both scientific research and daily routines. These reactions are responsible for maintaining life processes, supporting industries, and explaining natural phenomena. Without them, life as we know it would be impossible, and technological advancements would come to a standstill.
In everyday activities such as cooking, cleaning, and driving, chemical reactions occur constantly. In scientific fields, they are critical in pharmaceutical development, materials science, agriculture, and environmental management.
Overview of What the Article Will Cover
This article will provide a comprehensive understanding of chemical reactions by exploring their definitions, types, and essential features. It will explain how chemical equations are balanced, the factors that affect the speed of reactions, and the role of catalysts and enzymes in facilitating these changes.
Further, it will describe the differences between catalysts and inhibitors, energy changes associated with chemical reactions, and real-world examples of reactions happening all around us. Safety precautions while handling chemical reactions and answers to frequently asked questions will also be included.
What is a Chemical Reaction?
Chemical reactions involve the transformation of one or more substances into different products through the breaking and forming of chemical bonds. This process results in a change in the chemical composition and physical properties of the original materials.
Understanding chemical reactions helps explain both natural processes and man-made procedures in a wide range of scientific and practical contexts.
Definition of a Chemical Reaction
A chemical reaction is a process in which reactants interact to form products, involving the rearrangement of atoms and the breaking or formation of bonds. It is usually accompanied by observable changes such as energy release, color change, or the formation of a gas or precipitate.
These reactions occur naturally or can be induced in laboratories and industries to manufacture products or study material behavior.
Real-Life Examples of Chemical Reactions
Many familiar processes involve chemical reactions. Examples include the burning of fuels for heat and energy, rusting of iron when exposed to moisture, and photosynthesis in plants, where sunlight converts water and carbon dioxide into glucose and oxygen.
Inside the human body, countless reactions regulate processes like digestion, respiration, and immune responses.
Characteristics of a Chemical Reaction
Several distinct features define a chemical reaction. New substances with different properties from the reactants are formed, and there is often an observable physical or chemical change such as heat, light, or color.
Another essential characteristic is adherence to the law of conservation of mass, where the total mass of reactants equals the total mass of products. The speed and conditions under which these changes occur vary with each reaction.
Types of Chemical Reactions
Chemical reactions can be classified into different categories based on how reactants are transformed into products. These types are determined by the nature of bond rearrangements and the kinds of substances involved in the process. Understanding these types allows scientists to predict reaction behavior and outcomes under various conditions.
Below are the major types of chemical reactions commonly observed in both natural and industrial processes.
Synthesis (Combination) Reactions
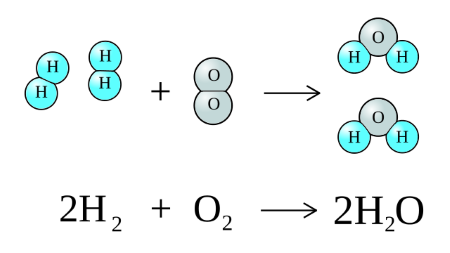
A synthesis reaction occurs when two or more reactants combine to form a single product. These reactions usually involve elements or simple compounds producing more complex substances.
An example of a synthesis reaction is the formation of water when hydrogen gas reacts with oxygen gas. Synthesis reactions are important in biological processes and chemical manufacturing where new compounds are assembled from simpler materials.
Decomposition Reactions
In decomposition reactions, a single compound breaks down into two or more simpler substances. This can happen through heating, application of electricity, or chemical catalysts.
An example is the breakdown of calcium carbonate into calcium oxide and carbon dioxide when heated. Decomposition reactions are important in metal extraction, waste treatment, and energy production processes.
Single Replacement Reactions
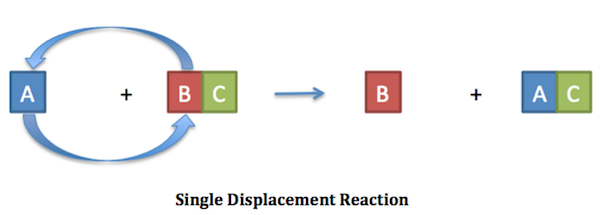
Single replacement reactions involve one element replacing another in a compound. A more reactive element displaces a less reactive element from its compound.
An example is when zinc metal reacts with copper sulfate solution, displacing copper to form zinc sulfate. These reactions are common in corrosion processes, metal purification, and electrochemical cells.
Double Replacement (Displacement) Reactions
Double replacement reactions occur when two compounds exchange their ions to form two new compounds. These reactions typically occur in aqueous solutions and often result in the formation of a precipitate, gas, or water.
An example is the reaction between silver nitrate and sodium chloride solutions, which produces silver chloride precipitate and sodium nitrate solution. This type of reaction is widely used in analytical chemistry, medicine, and water treatment.
Combustion Reactions
Combustion reactions involve the rapid reaction of a substance with oxygen, producing heat and light. They typically involve hydrocarbons reacting with oxygen to form carbon dioxide and water.
A common example is the burning of petrol in car engines. Combustion reactions are vital for energy generation, cooking, heating, and industrial applications.
Redox (Oxidation-Reduction) Reactions
Redox reactions involve the transfer of electrons between two substances. In these reactions, one substance is oxidized (loses electrons) while another is reduced (gains electrons).
A typical example is the rusting of iron, where iron is oxidized, and oxygen is reduced. Redox reactions are essential in biological metabolism, electrochemical processes, and industrial extraction of metals.
Balancing Chemical Reactions
Balancing chemical reactions is an essential skill in chemistry, ensuring that the chemical equation reflects the conservation of mass. It involves adjusting the quantities of reactants and products so that the number of each type of atom remains the same on both sides of the equation.
This process is necessary for accurate stoichiometric calculations and for understanding how reactions proceed quantitatively.
Why Balancing Chemical Equations is Important
Balancing chemical equations ensures that no atoms are lost or gained during a reaction, aligning with the law of conservation of mass. It provides a correct quantitative relationship between reactants and products, which is vital for laboratory work and industrial applications.
An unbalanced equation could lead to errors in predicting product amounts, resource requirements, and reaction yields.
Law of Conservation of Mass
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. This means the total mass of the substances before and after a chemical reaction must be the same.
Balancing chemical equations ensures compliance with this principle by adjusting the number of each atom type on both sides of the reaction without changing the compounds involved.
Steps for Balancing Chemical Equations
To balance a chemical equation, first identify and count the number of atoms of each element on both sides of the equation. Start balancing elements that appear in only one reactant and one product. Then, balance elements appearing in multiple compounds.
It’s advisable to leave hydrogen and oxygen atoms for last as they often appear in multiple places. Finally, verify that all elements are balanced and simplify coefficients to the smallest whole numbers if possible.
Common Mistakes and How to Avoid Them
A common error when balancing equations is changing the chemical formula (subscripts) instead of adjusting coefficients. Another mistake is failing to balance polyatomic ions as a group when they remain unchanged on both sides.
To avoid these issues, double-check atom counts after each adjustment and work systematically from simple to more complex elements.
Factors Affecting Chemical Reactions
The rate and outcome of chemical reactions are influenced by several factors. These factors determine how quickly reactants are converted into products and whether the reaction is efficient and complete under certain conditions. Understanding these influences allows scientists and industries to control reaction environments for desired results.
The most significant factors include temperature, concentration, surface area, presence of catalysts or inhibitors, pressure in gaseous systems, and the nature of the reactants involved.
Temperature
Temperature has a direct impact on reaction rates because it affects the kinetic energy of particles. Increasing temperature causes particles to move faster, increasing the frequency and energy of collisions.
As a result, reactions generally proceed more quickly at higher temperatures. However, extremely high temperatures may also lead to undesirable side reactions or the breakdown of sensitive compounds.
Concentration of Reactants
The concentration of reactants in a solution or mixture influences how often particles collide. A higher concentration means more particles are present in a given volume, increasing the likelihood of collisions and, consequently, the reaction rate.
This principle is particularly important in laboratory and industrial reactions, where adjusting reactant concentrations can significantly alter production yields and processing times.
Surface Area of Reactants
When at least one reactant is a solid, its surface area affects how readily it reacts. Finely divided or powdered substances have a greater surface area exposed to other reactants, resulting in faster reactions.
This is why combustible powders ignite more readily than larger solid blocks and why catalysts are often used in powdered form to maximize efficiency.
Presence of Catalysts and Inhibitors
Catalysts speed up chemical reactions by providing an alternative pathway with lower activation energy, while inhibitors slow reactions by interfering with reactant interactions.
Catalysts are widely used in industrial processes, environmental controls, and biological systems, while inhibitors are crucial for controlling polymerization reactions or preventing unwanted changes in storage and processing.
Pressure (for Gaseous Reactions)
In reactions involving gases, increasing the pressure compresses the gas molecules into a smaller volume, raising their concentration and increasing the frequency of collisions.
This factor is essential in processes like the Haber process for ammonia synthesis, where high pressures are used to drive the reaction toward higher product yields.
Nature of Reactants
The inherent chemical properties of the substances involved affect reaction speed. Ionic compounds in aqueous solutions tend to react faster than covalent compounds due to the free movement of ions.
The complexity of molecular bonds, bond strengths, and reactivity series of elements also contribute to how readily reactants undergo chemical change.
Reaction Rates and How to Measure Them
The speed at which a chemical reaction proceeds, known as the reaction rate, is a critical parameter in chemical processes. It indicates how fast reactants are transformed into products and is influenced by the factors discussed previously.
Measuring reaction rates is vital in both research and industry for optimizing conditions, ensuring safety, and maximizing efficiency.
Definition of Reaction Rate
The reaction rate is defined as the change in concentration of a reactant or product per unit time. It is usually expressed in moles per liter per second (mol/L/s) or as a change in mass or volume over time for certain reactions.
A fast reaction occurs within seconds, like combustion, while a slow one may take hours or days, like the rusting of iron.
Units of Reaction Rates
Reaction rates can be expressed in various units depending on how the reaction progress is monitored. Common units include mol/L/s for concentration changes, grams per minute for mass changes, or milliliters per second for volume changes in gas-producing reactions.
The chosen unit must match the method used to observe the reaction and the quantities involved.
Factors Influencing Reaction Rates
The same factors discussed earlier temperature, concentration, surface area, presence of catalysts, pressure, and the nature of reactants affect how quickly a reaction proceeds.
By understanding how these variables influence reaction rates, scientists can manipulate conditions to achieve desired outcomes in laboratory and industrial settings.
Methods for Measuring Reaction Rates
Several practical techniques exist for measuring how fast a reaction proceeds. The choice of method depends on whether the reaction involves a change in mass, volume, color, or electrical conductivity.
These measurements help scientists monitor reaction progress and understand the underlying reaction kinetics.
Change in Mass Over Time
In reactions that produce a gas, the loss of mass from a reaction vessel can be measured over time. This method is simple, requiring only a balance and timer, and is commonly used in acid-carbonate reactions.
By plotting mass against time, the reaction rate can be determined from the slope of the graph.
Change in Volume of Gas
For reactions that release gas, measuring the volume of gas collected over time provides a reliable rate measurement. Devices such as gas syringes or inverted measuring cylinders are typically used.
This method is suitable for reactions like the decomposition of hydrogen peroxide or metal-acid reactions.
Colorimetric Changes
When a reaction involves a noticeable color change, a colorimeter or spectrophotometer can track changes in light absorption over time. This is especially useful in reactions involving dyes, indicators, or biological pigments.
By correlating absorbance readings with concentration, reaction rates can be accurately monitored.
Conductivity Measurements
In reactions that produce or consume ions, measuring changes in electrical conductivity provides a quick indication of reaction progress. This technique is useful in acid-base titrations and electrochemical reactions.
Specialized conductivity meters continuously track the ionic content of the solution, providing data on reaction speed.
Applications of Reaction Rate Studies
Understanding reaction rates is crucial in various fields where chemical processes must be optimized for safety, efficiency, and cost-effectiveness. Studying these rates enables the development of better products, sustainable processes, and controlled reaction environments.
Below are some key application areas.
Industrial Manufacturing Processes
Reaction rate control is vital in industrial manufacturing to maximize product yields, minimize waste, and improve production timelines. Faster reactions enhance efficiency, while controlled slower rates may be needed to maintain product quality.
Examples include fertilizer production, plastics manufacturing, and refining processes.
Pharmaceutical Drug Development
In the pharmaceutical industry, reaction rates determine how quickly drugs are metabolized and how stable they remain during storage. Understanding these rates ensures medication effectiveness, appropriate dosage forms, and shelf life.
It also aids in the development of slow-release formulations for sustained therapeutic effects.
Environmental Chemistry
Reaction rate studies are critical in understanding how pollutants break down in air, water, and soil. This knowledge helps design effective waste treatment and pollution control strategies.
It also assists in predicting the environmental impact of chemicals and in developing eco-friendly alternatives.
Food Preservation and Processing
Controlling reaction rates is essential in food science for preventing spoilage and ensuring safety. Techniques like refrigeration, vacuum sealing, and adding preservatives slow down microbial and enzymatic reactions.
Conversely, certain processes like fermentation require controlled reaction acceleration for product formation.
Catalysts in Chemical Reactions
Catalysts play an essential role in altering the speed of chemical reactions without being consumed in the process. These substances work by providing an alternative reaction pathway that requires less activation energy, thereby increasing the rate at which products form.
Understanding catalysts is crucial in both industrial manufacturing and biological systems, as they enable reactions to occur efficiently under milder conditions.
Definition and Role of Catalysts
A catalyst is a substance that increases the rate of a chemical reaction without undergoing permanent chemical change itself. It achieves this by lowering the activation energy required for the reaction to proceed.
Catalysts are used extensively in industries to make reactions more economical, reduce energy consumption, and enhance product yields. In biological systems, enzymes serve as natural catalysts that sustain life processes.
Examples of Catalytic Reactions
Several practical reactions demonstrate the importance of catalysts. For example, in vehicle exhaust systems, platinum catalysts accelerate the breakdown of harmful gases like carbon monoxide into less toxic substances.
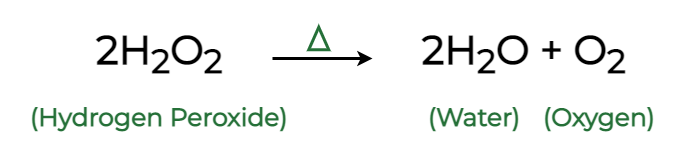
In industrial chemistry, the Haber process uses an iron catalyst to produce ammonia from nitrogen and hydrogen. Similarly, enzymes like catalase rapidly decompose hydrogen peroxide into water and oxygen inside living cells, protecting tissues from oxidative damage.
Differences Between Catalysts and Inhibitors
While catalysts speed up reactions, inhibitors have the opposite effect by slowing them down or halting them entirely. Catalysts lower activation energy, making it easier for reactants to form products, while inhibitors either increase activation energy or block reactive sites on molecules.
Inhibitors are useful in situations where it is necessary to control the rate of a reaction, such as in preserving materials, controlling biological processes, or preventing corrosion.
Enzymes: Nature’s Catalysts
Enzymes are biological catalysts made of proteins or RNA that increase the rate of biochemical reactions without being consumed. They are highly specific, usually catalyzing only one type of reaction or interacting with a particular substrate.
These natural catalysts are essential for life, driving processes like digestion, cellular respiration, and DNA replication under mild physiological conditions.
What Are Enzymes?
Enzymes are complex biomolecules, primarily proteins, with three-dimensional structures that allow them to bind specifically to their substrates. The region where this binding occurs is called the active site.
After facilitating the reaction, the enzyme releases the product and returns to its original state, ready to catalyze subsequent reactions. Their precision ensures that vital biochemical processes proceed efficiently without unintended side effects.
How Enzymes Speed Up Biochemical Reactions
Enzymes speed up reactions by stabilizing the transition state and lowering the activation energy. They achieve this by correctly positioning substrates, providing reactive chemical groups, and creating an optimal microenvironment for bond formation or cleavage.
This mechanism allows reactions that would otherwise be too slow under biological conditions to proceed rapidly, supporting the continuous metabolism required for life.
Factors Influencing Enzyme Activity
Several factors affect enzyme performance. Temperature plays a significant role; most enzymes function best within a narrow temperature range, beyond which they may denature or become inactive.
pH is another critical factor, as changes in acidity or alkalinity can alter the enzyme’s shape and its ability to bind substrates. Additionally, substrate concentration, presence of activators, and inhibitors influence enzyme activity by affecting reaction rates and efficiency.
Energy Changes in Chemical Reactions
Every chemical reaction involves a change in energy as bonds are broken and new ones formed. These energy changes determine whether a reaction absorbs or releases heat, influencing its feasibility and practical applications.
Reactions are broadly classified into exothermic and endothermic based on the direction of energy transfer.
Exothermic vs Endothermic Reactions
Exothermic reactions release energy, usually as heat or light, into their surroundings. Common examples include combustion, respiration, and neutralization reactions, which are typically spontaneous and result in a temperature increase.
Endothermic reactions, in contrast, absorb energy from the environment, often leading to a temperature drop. Photosynthesis and the thermal decomposition of calcium carbonate are classic endothermic processes requiring continuous energy input to proceed.
Activation Energy Explained
Activation energy is the minimum amount of energy required for reactants to undergo a transformation and form products. It represents the barrier that must be overcome for a reaction to initiate.
Catalysts lower the activation energy by providing an alternative reaction pathway, thereby allowing reactions to proceed more quickly and under less severe conditions than would otherwise be possible.
Energy Diagrams and Their Interpretation
Energy diagrams graphically represent the energy changes during a reaction, showing the energy levels of reactants, the peak representing activation energy, and the final energy level of the products.
In an exothermic reaction, the products have lower energy than the reactants, while in an endothermic reaction, the products’ energy is higher. These diagrams help predict whether a reaction will proceed spontaneously and illustrate the effects of catalysts on activation energy.
Real-World Examples of Chemical Reactions
Chemical reactions are integral to everyday experiences and industrial operations. Recognizing these reactions helps connect theoretical concepts with practical applications and highlights the importance of chemistry in solving real-world problems.
Several well-known examples illustrate this relevance.
Combustion of Fuels
Combustion is a rapid exothermic reaction between a substance and oxygen, producing heat and light. It typically involves hydrocarbons reacting with oxygen to yield carbon dioxide and water.
This reaction is the basis for energy production in engines, heating systems, and industrial furnaces. Despite its benefits, combustion also contributes to air pollution and greenhouse gas emissions.
Photosynthesis in Plants
Photosynthesis is an essential endothermic reaction where green plants convert carbon dioxide and water into glucose and oxygen, using sunlight as an energy source. Chlorophyll captures light energy, driving the reaction within plant cells.
This process sustains life on Earth by producing food for organisms and maintaining atmospheric oxygen levels.
Rusting of Iron
Rusting is a slow redox reaction in which iron reacts with oxygen and moisture to form hydrated iron(III) oxide, commonly known as rust. This process weakens metal structures and leads to significant economic losses in construction, transportation, and manufacturing.
Preventative measures include applying protective coatings, using corrosion-resistant alloys, and introducing corrosion inhibitors.
Decomposition of Baking Soda
When baking soda (sodium bicarbonate) is heated, it decomposes into sodium carbonate, water vapor, and carbon dioxide gas. This reaction is utilized in baking to produce gas bubbles that make dough and batter rise.
It also finds use in fire extinguishers, personal care products, and cleaning agents due to its mild abrasive and deodorizing properties.
Safety Precautions in Handling Chemical Reactions
Although chemical reactions offer valuable benefits, they also present risks if mishandled. Implementing safety precautions ensures personal protection, environmental safety, and the success of experimental procedures.
Proper laboratory practices, careful handling of hazardous chemicals, and appropriate use of protective equipment are essential in any setting where chemical reactions occur.
Proper Laboratory Practices
Maintaining a clean and organized workspace prevents contamination and accidents. It’s important to label all containers clearly, use appropriate glassware and equipment, and follow established safety protocols.
Working under fume hoods when dealing with volatile or hazardous substances, and keeping flammable materials away from ignition sources, minimizes risks in laboratory environments.
Handling of Hazardous Chemicals
Some chemicals are corrosive, toxic, or highly reactive. They must be stored, handled, and disposed of according to strict safety guidelines. Consulting material safety data sheets (MSDS) provides essential information on potential hazards and emergency procedures.
Workspaces should be equipped with spill containment kits, eyewash stations, and emergency showers to manage accidental exposures.
Use of Personal Protective Equipment (PPE)
Personal protective equipment, such as lab coats, gloves, goggles, and face shields, provides a physical barrier against chemical splashes, burns, and harmful vapors. In certain cases, additional gear like respirators and protective footwear is necessary.
Regular inspection and maintenance of PPE ensure effective protection during routine and high-risk operations.
Conclusion
Chemical reactions underpin virtually every process in both nature and human-made systems. From biological metabolism to industrial production and environmental processes, understanding chemical reactions is fundamental to advancing science and technology.
By studying reaction types, balancing equations, controlling reaction rates, and recognizing energy changes, scientists can design safer, more efficient, and environmentally friendly processes. As research continues, future developments in reaction control and sustainable chemistry will play a crucial role in addressing global challenges.
Frequently Asked Questions (FAQ)
What is the easiest way to balance chemical equations?
The easiest way is to balance atoms one element at a time using coefficients, starting with the most complex molecule and leaving hydrogen and oxygen for last.
Which factors most significantly affect reaction rates?
Reaction rates are mainly affected by temperature, concentration of reactants, surface area, and the presence of a catalyst.
What is the difference between a catalyst and an enzyme?
A catalyst speeds up a chemical reaction without being consumed, while an enzyme is a biological catalyst that specifically speeds up reactions in living organisms.
Related Contents
Chargaff’s Rules: First and Second Rules, Applications
Centromere- Definition, Structure, Position, Types, Functions