Centromeres are fundamental components of chromosomes, playing a crucial role in ensuring the accurate segregation of genetic material during cell division. Although often overlooked in comparison to genes themselves, the centromere’s structural and functional integrity is vital for chromosomal stability and inheritance. Recent research has highlighted its dynamic nature, regulatory complexity, and importance in human disease and evolution.
This content provides an in-depth explanation of the centromere, exploring its definition, structure, position, and biological significance. It also addresses the disorders associated with centromere dysfunction, current research tools, and therapeutic prospects involving centromere biology.
Summary of Centromere
- The centromere is a specialized region on a chromosome that ensures accurate separation of chromosomes during cell division by forming the kinetochore, where spindle fibers attach.
- Its structure is defined not just by DNA sequences but also by unique proteins like CENP-A, making it essential for chromosome stability and proper segregation.
- Defects in centromere function can lead to chromosomal abnormalities such as aneuploidy, contributing to genetic diseases, cancer, and developmental disorders.
Table of Contents
Introduction
In the complex world of cellular biology, precise mechanisms are required to ensure that genetic material is equally distributed to daughter cells. One such essential mechanism is facilitated by the centromere. Without this structure, chromosomes would fail to align and separate properly, leading to genetic instability, cell death, or diseases such as cancer.
The centromere’s role extends beyond mere chromosomal attachment—it orchestrates a sophisticated molecular choreography that governs cell division and inheritance.
Importance of the Centromere in Cell Division
The centromere is indispensable for ensuring the faithful segregation of chromosomes during both mitosis and meiosis. It serves as the attachment site for the kinetochore, a protein complex essential for binding chromosomes to spindle microtubules. This connection is vital for equal distribution of duplicated chromosomes to daughter cells.
Any malfunction in centromere structure or function can lead to aneuploidy, a condition where cells possess an abnormal number of chromosomes, resulting in developmental disorders, infertility, or malignancies.
Historical Discovery and Biological Significance
The centromere was first observed in the 19th century by cytologists using primitive microscopes, who noticed a constricted region on metaphase chromosomes where spindle fibers attached. As cytogenetics progressed, the biological importance of this region became clearer.
Modern research reveals that the centromere not only anchors kinetochores but also maintains chromosomal identity and structure across generations, making it a cornerstone of genomic stability and evolution.
What is a Centromere?
Definition of Centromere
A centromere is a specific chromosomal region essential for the proper segregation of chromosomes during cell division. It serves as the site for kinetochore formation and spindle fiber attachment. Functionally, it ensures that sister chromatids remain linked until they are ready to be separated into daughter cells.
Its precise position varies across species and chromosomes but its role in chromosomal movement and alignment is universal among eukaryotes.
Etymology and Historical Background
The term “centromere” is derived from the Greek words kentron (center) and meros (part), originally describing the central position it was thought to occupy. Historically, centromeres were identified as the primary constriction on chromosomes visible during metaphase under a light microscope.
As microscopy techniques improved, this region was recognized as the attachment site for spindle fibers, and molecular studies later identified its unique DNA sequences and associated proteins.
Structure of the Centromere
Although appearing as a simple constriction under a microscope, the centromere is a complex, multilayered structure composed of specialized DNA sequences, chromatin, and protein assemblies.
Key Components
DNA Sequences (Alpha Satellite DNA)
In humans and many other species, centromeres contain long stretches of repetitive DNA known as alpha satellite DNA. These sequences are highly conserved within a species but can vary between different species. Though their exact functional contribution remains under study, they are essential for centromere identity and kinetochore formation.
The repetitive nature of these sequences aids in the binding of centromere-specific proteins, facilitating the organization of the centromeric chromatin.
Centromeric Chromatin
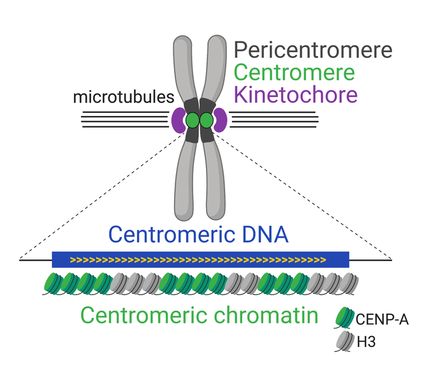
The DNA within the centromere is packaged into a distinct type of chromatin called centromeric chromatin. Unlike typical chromatin, it incorporates a histone H3 variant known as CENP-A, which marks the centromeric region epigenetically and is crucial for kinetochore assembly.
This specialized chromatin ensures that the centromere is maintained at the same location after each round of DNA replication, despite the lack of a strict DNA sequence requirement in some species.
Kinetochore Formation
The kinetochore is a multiprotein complex that assembles on the centromere during cell division. It serves as a platform for spindle fiber attachment and coordinates the movement of chromosomes to opposite poles of the dividing cell.
Kinetochore proteins interact with CENP-A containing nucleosomes, ensuring correct microtubule attachment and regulating the timing of chromatid separation.
Epigenetic Factors Influencing Centromere Identity
Recent research highlights the role of epigenetic mechanisms, particularly the deposition of CENP-A and specific histone modifications, in defining centromere location. Even in the absence of typical alpha satellite DNA, these epigenetic markers can establish and maintain centromeric function.
This suggests that centromere identity is not solely dictated by DNA sequence but also by chromatin state and associated protein complexes.
Position of the Centromere on Chromosomes
Centromeres can occupy different positions along the length of a chromosome, affecting its morphology and behavior during cell division.
Chromosome Morphology Based on Centromere Location
Based on the position of the centromere, chromosomes are categorized into four morphological types: metacentric, submetacentric, acrocentric, and telocentric. This classification aids cytogeneticists in identifying chromosomes and diagnosing chromosomal abnormalities.
The location influences how chromosomes behave during segregation and how forces are distributed by the spindle apparatus.
How Position Affects Genetic Behavior
The centromere’s location impacts the mechanical tension during metaphase alignment and anaphase separation. Chromosomes with terminal centromeres (telocentric) behave differently compared to those with centrally located centromeres (metacentric), potentially affecting segregation fidelity.
Aberrant positioning or structural alterations involving centromeres can disrupt normal genetic inheritance and lead to aneuploidy or chromosomal instability syndromes.
Types of Centromeres
Based on Position
Metacentric
In metacentric chromosomes, the centromere lies in the middle, dividing the chromosome into two equal arms. During anaphase, these chromosomes form a distinctive “V” shape as they move to opposite poles.
Submetacentric
Here, the centromere is slightly off-center, producing one arm longer than the other. These chromosomes adopt an “L” shape during anaphase.
Acrocentric
In acrocentric chromosomes, the centromere is located close to one end, creating a very short arm and a long arm. Humans possess five pairs of acrocentric chromosomes, which play roles in ribosomal RNA production.
Telocentric
These chromosomes have the centromere at the very end. They are common in certain animal species but do not occur naturally in humans.
Holocentric Chromosomes: An Exception
Holocentric chromosomes lack a localized centromere. Instead, spindle fibers attach along the entire length of the chromosome. Found in some plants, insects, and nematodes, these chromosomes challenge traditional models of chromosome segregation and open new avenues in centromere research.
Functions of the Centromere
The centromere performs several essential cellular functions beyond serving as a physical constriction on chromosomes.
Ensuring Accurate Chromosome Segregation
Its primary function is to guarantee the precise segregation of sister chromatids during cell division. This prevents the gain or loss of chromosomes, maintaining genomic integrity in daughter cells.
Kinetochore Assembly and Microtubule Attachment
Centromeres act as assembly sites for kinetochores, which physically connect chromosomes to the mitotic spindle. The stability and proper orientation of these attachments determine the success of chromosome separation.
Role in Mitosis and Meiosis
During mitosis, centromeres ensure equal genetic distribution, while in meiosis, they contribute to the reductional division process. Accurate segregation during meiosis is vital for fertility and genetic diversity.
Maintaining Chromosome Stability
Centromeres play a structural role in preserving chromosomal architecture. They prevent chromosome breakage, fusion, and loss, ensuring the stability of the genome through multiple cell generations.
Mechanisms of Centromere Replication and Inheritance
The replication and inheritance of centromeres are tightly regulated processes that ensure each daughter cell receives a functional centromere. Despite the repetitive nature of centromeric DNA, its duplication and the retention of centromeric identity across cell divisions require a combination of DNA replication and epigenetic mechanisms. These ensure that centromeres are faithfully propagated even when DNA sequences alone are insufficient to specify centromere location.
DNA Replication of Centromeric Regions
Centromeric DNA is replicated during the late S phase of the cell cycle, slightly later than other genomic regions. This timing is thought to contribute to the specialized chromatin structure of the centromere. The repetitive sequences pose unique challenges for DNA polymerases, often requiring specialized replication machinery and proteins to prevent replication errors.
Failure to accurately replicate these regions can lead to centromere dysfunction, resulting in errors in chromosome segregation and genomic instability.
Centromeric Histone Variants (CENP-A)
A defining feature of centromeric chromatin is the presence of the histone H3 variant known as CENP-A. This histone replaces conventional H3 within nucleosomes at the centromere and acts as an epigenetic marker that specifies centromere identity.
The deposition of CENP-A is independent of DNA replication and typically occurs in early G1 phase after cell division. Specialized chaperone proteins, such as HJURP in humans, assist in the targeted incorporation of CENP-A into centromeric nucleosomes. Without proper CENP-A assembly, kinetochore formation fails, and chromosomal segregation is compromised.
Diseases and Disorders Linked to Centromere Dysfunction
Centromeres play such a critical role in cell division that their malfunction can have serious biological consequences. A range of human diseases arises from centromere defects, primarily through mechanisms involving chromosomal instability or segregation errors.
Aneuploidy and Cancer
Errors in centromere function are a major cause of aneuploidy, a condition where cells possess an abnormal number of chromosomes. This is frequently observed in cancer cells, where disrupted chromosome segregation leads to genetic imbalances that fuel tumor development and progression.
Certain aggressive cancers exhibit centromere amplification, mislocalization, or loss, highlighting the direct link between centromere integrity and oncogenesis.
Centromere Instability Syndromes
Rare genetic syndromes such as ICF (Immunodeficiency, Centromeric instability, and Facial anomalies) syndrome are directly associated with defective centromeric regions. These disorders are characterized by abnormal chromosomal structures, immunodeficiency, and developmental anomalies due to improper centromere organization and replication.
Such conditions underscore the importance of centromere maintenance for human health.
Congenital Disorders Linked to Centromere Defects
Certain congenital disorders, including some forms of microcephaly and infertility, have been linked to mutations in centromere-associated proteins. Mis-segregation of chromosomes during early embryonic divisions can result in developmental defects, miscarriages, or genetic syndromes such as Down syndrome.
These findings demonstrate that centromere abnormalities can influence not just somatic cells but also germline and embryonic development.
Techniques to Study Centromeres
To understand centromere biology and its role in disease, researchers rely on a variety of sophisticated molecular and cytogenetic techniques. These tools enable the visualization, isolation, and functional analysis of centromeric regions.
Fluorescence In Situ Hybridization (FISH)
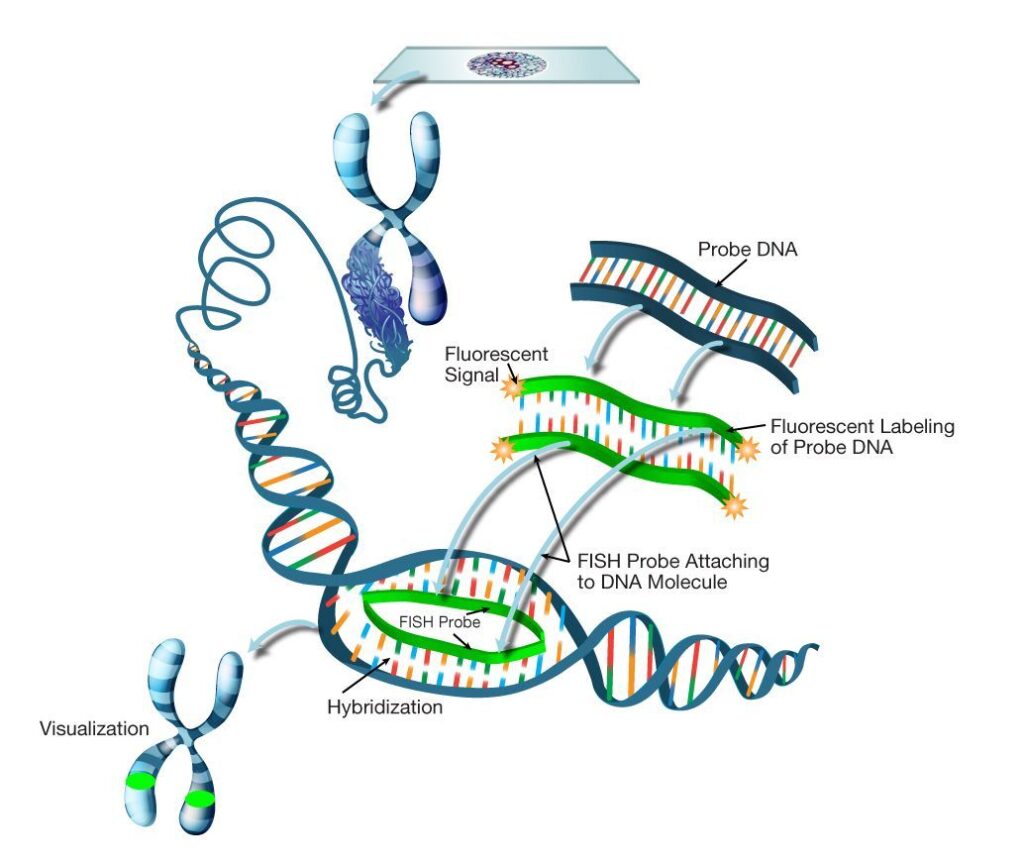
FISH is a widely used technique that involves hybridizing fluorescently labeled DNA probes to specific centromeric sequences. This allows direct visualization of centromeres on metaphase chromosomes under a fluorescence microscope.
FISH is invaluable in detecting aneuploidies, identifying chromosomal abnormalities, and studying centromere organization in both clinical and research settings.
Chromatin Immunoprecipitation (ChIP)
ChIP is a molecular technique that isolates DNA sequences bound by specific proteins. When combined with antibodies against CENP-A or other centromeric proteins, ChIP enables researchers to map active centromeres and study the chromatin environment surrounding them.
This method has been essential for understanding the epigenetic regulation of centromere identity and kinetochore assembly.
Centromere-Specific Protein Markers
Proteins such as CENP-A, CENP-C, and CENP-E are centromere-specific markers that help in identifying and characterizing functional centromeres. Antibodies against these proteins are used in immunostaining, ChIP assays, and proteomic analyses.
The study of these markers provides insights into the composition and dynamics of the kinetochore complex and its role in chromosome segregation.
Evolutionary Aspects of Centromeres
Centromeres are among the most rapidly evolving genomic regions, showing significant variation in DNA sequence even among closely related species. This has intriguing implications for species divergence and genome evolution.
Rapid Evolution of Centromeric DNA
Centromeric DNA evolves at a fast pace due to its repetitive nature and tolerance for sequence variation. Despite this, the epigenetic marks defining centromeres remain remarkably conserved.
The disparity between DNA sequence variation and functional stability illustrates the importance of epigenetic regulation in centromere identity.
Adaptive Significance in Speciation
Centromeres can act as barriers to hybridization between species by contributing to meiotic errors in hybrid offspring. Changes in centromere-associated sequences or proteins can lead to reproductive isolation, driving speciation.
This suggests that centromere evolution plays an active role in the diversification of species through its impact on fertility and genome compatibility.
Centromeres in Modern Research and Therapeutics
As our understanding of centromere biology deepens, new opportunities have emerged to exploit this knowledge in biotechnology and medicine. Artificial chromosome construction, gene therapy vectors, and regenerative medicine all involve centromere engineering.
Artificial Chromosomes in Gene Therapy
Artificial chromosomes containing functional centromeres have been developed as potential vectors for gene therapy. These synthetic chromosomes can carry large genetic payloads and stably replicate in human cells without integrating into existing chromosomes, reducing the risk of insertional mutagenesis.
Such artificial chromosomes could provide new avenues for treating genetic diseases by delivering functional genes without disturbing native chromosomal structures.
Centromere Role in Stem Cell Biology
Stem cells possess unique cell division dynamics, and maintaining centromere integrity is crucial for their self-renewal and pluripotency. Errors in centromere function in stem cells can lead to genomic instability, differentiation defects, or tumorigenesis.
Understanding centromere behavior in stem cells has implications for regenerative medicine, aging research, and cancer biology.
Ethical Considerations in Centromere Engineering
With advancements in genetic manipulation, ethical concerns arise about the potential consequences of modifying centromeric regions. The creation of artificial chromosomes or genome editing near centromeres could have unforeseen effects on chromosomal behavior and inheritance.
Genetic Editing and Chromosomal Modifications
Targeting centromeric regions for therapeutic or research purposes requires caution due to their essential role in genome stability. Unintended alterations could result in aneuploidy or gene disruption, emphasizing the need for stringent oversight.
Ethical guidelines advocate for comprehensive risk assessments and regulatory controls before implementing centromere engineering technologies in clinical or agricultural contexts.
Regulatory Oversight in Therapeutic Applications
International and national bioethics committees emphasize the need for strict regulatory frameworks governing centromere modification technologies. This ensures that benefits are balanced against potential risks, safeguarding human health and genetic diversity.
Ongoing debates address the acceptability of inheritable chromosomal modifications and the implications for future generations.
Conclusion
Centromeres are essential for the faithful transmission of genetic material, ensuring chromosome stability and equal segregation during cell division. Their intricate structure, governed by both DNA sequence and epigenetic mechanisms, exemplifies the complexity of genomic regulation.
Despite their structural conservation, centromeres remain dynamic evolutionary elements influencing speciation, disease, and cellular function. As research tools and therapeutic strategies continue to evolve, centromeres hold promise in fields ranging from gene therapy to regenerative medicine.
Future research is expected to uncover deeper insights into centromere biology, particularly in understanding holocentric chromosomes, artificial chromosome applications, and the role of centromeres in age-related diseases and cancer. The ethical and regulatory frameworks guiding these innovations will shape the responsible application of centromere-based biotechnologies in human health and beyond.
Frequently Asked Questions (FAQ)
What determines the position of a centromere?
The position of a centromere is determined by specific DNA sequences and epigenetic markers, particularly the presence of the centromeric histone variant CENP-A.
How do centromeres differ between species?
Centromeres vary in DNA sequence and size across species, but their function is conserved; some species have single regional centromeres, while others, like certain insects and plants, have holocentric chromosomes with centromeric activity along the entire length.
Can centromere malfunctions lead to diseases?
Yes centromere defects can cause improper chromosome segregation, leading to conditions like aneuploidy, cancers, infertility, and congenital disorders.