The discovery and use of antibiotics targeting bacterial cell walls marked a revolutionary milestone in modern medicine. These agents remain among the most important and widely used classes of antibiotics, especially for treating severe and life-threatening infections. Targeting the bacterial cell wall is particularly effective because human cells lack this structure, allowing selective toxicity toward pathogenic bacteria without harming host cells.
Bacteria require strong, rigid cell walls to maintain their shape, protect against osmotic pressure, and survive in various environmental conditions. The cell wall’s integrity is crucial for bacterial survival, and inhibiting its synthesis leads to cell lysis and death, making it a prime target for antimicrobial therapy.
Summary of Cell wall synthesis inhibitors
- Cell wall synthesis inhibitors are antibiotics that disrupt bacterial peptidoglycan formation, leading to cell lysis and death. Major examples include beta-lactams (penicillins, cephalosporins) and glycopeptides (vancomycin).
- They act by inhibiting enzymes involved in peptidoglycan cross-linking or precursor synthesis, compromising bacterial cell wall integrity without affecting human cells, making them highly selective and effective.
- Resistance mechanisms include beta-lactamase production, altered penicillin-binding proteins, and reduced drug permeability, prompting ongoing research into new inhibitors and combination therapies.
Table of Contents
What are Cell Wall Synthesis Inhibitors?
Definition and Biological Significance
Cell wall synthesis inhibitors are a group of antimicrobial agents that specifically block the formation of peptidoglycan, a vital structural component of bacterial cell walls. By disrupting peptidoglycan synthesis, these drugs compromise the cell’s structural integrity, leading to osmotic imbalance and cell lysis. These inhibitors are primarily effective against actively dividing bacterial cells since cell wall synthesis is most active during cell division.
The biological significance of these inhibitors lies in their selective toxicity. Since human cells do not possess cell walls or peptidoglycan, these drugs selectively target bacteria without affecting human cells, minimizing side effects and making them suitable for clinical use.
Historical Development of Cell Wall Inhibitors
The development of cell wall synthesis inhibitors began with the discovery of penicillin by Alexander Fleming in 1928. This groundbreaking discovery opened new avenues for antimicrobial therapy, particularly during World War II. Over subsequent decades, researchers isolated and developed several related compounds, collectively known as beta-lactams, which expanded the therapeutic options against various bacterial infections.
The introduction of glycopeptides like vancomycin in the 1950s addressed infections caused by Gram-positive organisms resistant to beta-lactams. Since then, additional agents such as fosfomycin, bacitracin, and cycloserine have been introduced, each targeting different stages or enzymes involved in cell wall biosynthesis. Continuous refinement and discovery of new inhibitors remain a priority due to the persistent threat of antibiotic resistance.
Mechanism of Action of Cell Wall Synthesis Inhibitors
Before understanding how these inhibitors work, it is essential to comprehend the structure and function of peptidoglycan, the primary target of these drugs.
Overview of Peptidoglycan Structure
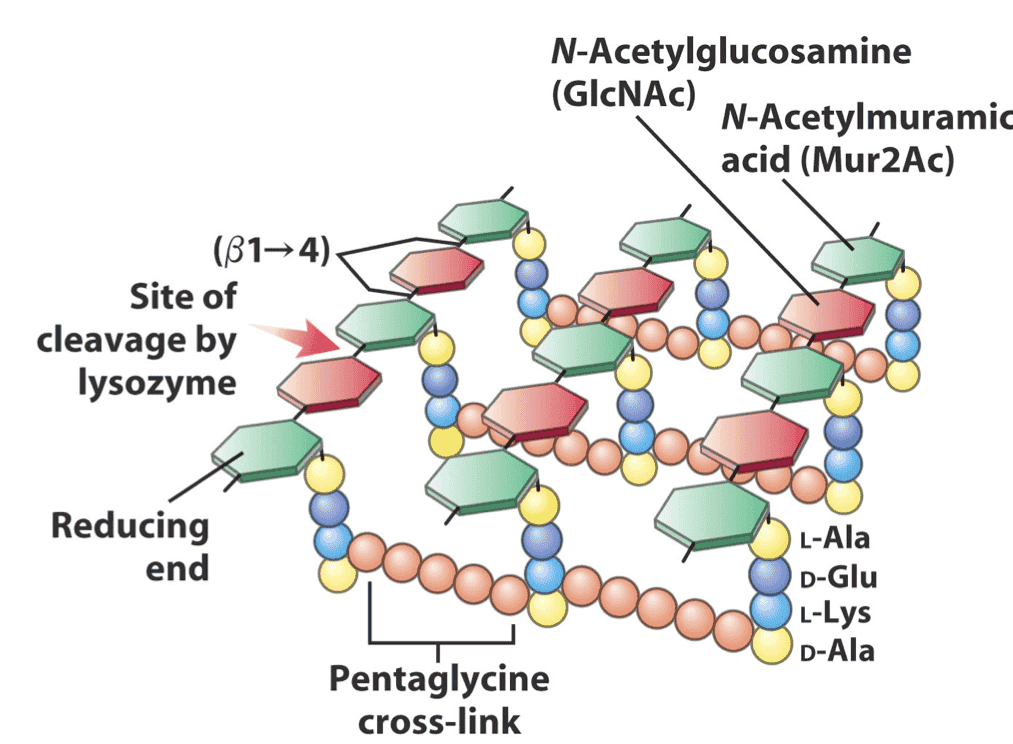
Peptidoglycan is a unique, mesh-like polymer composed of sugars and amino acids, forming a rigid layer surrounding the bacterial cell membrane. It consists of repeating units of two alternating sugars: N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM), linked by β-1,4 glycosidic bonds. Attached to each NAM unit is a short peptide chain, which cross-links with peptides from adjacent chains to form a strong, interconnected lattice.
This complex network provides mechanical strength, maintains cell shape, and protects against osmotic lysis. The continuous remodeling of this structure during bacterial growth and division makes it an accessible and vulnerable target for antimicrobial agents.
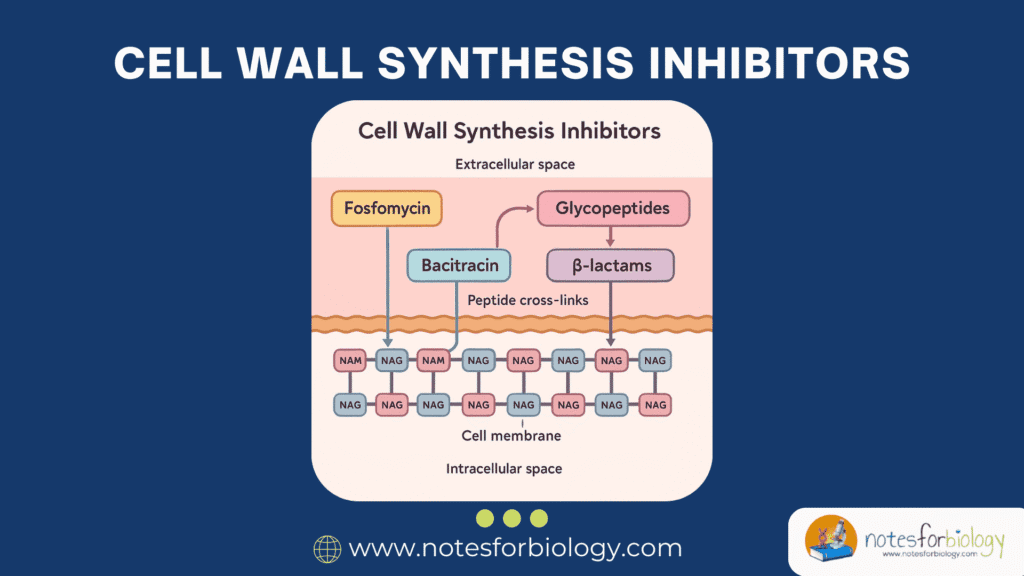
How Inhibitors Disrupt Peptidoglycan Cross-Linking
Most cell wall synthesis inhibitors act by preventing the formation of cross-links between the peptide chains of peptidoglycan, a process catalyzed by enzymes known as penicillin-binding proteins (PBPs). Beta-lactam antibiotics, for example, mimic the natural substrate of PBPs and irreversibly bind to their active sites, halting transpeptidation.
Glycopeptide antibiotics, like vancomycin, bind directly to the D-Ala-D-Ala termini of the peptide chains, physically blocking the access of PBPs and preventing cross-linking. Other agents, such as bacitracin and fosfomycin, interfere with earlier stages of peptidoglycan precursor formation and transport.
Impact on Bacterial Viability
By compromising the structural integrity of the bacterial cell wall, these inhibitors create weaknesses in the peptidoglycan layer, leading to osmotic instability. Bacteria typically exist in hypotonic environments, where water tends to enter the cell. Without a rigid cell wall to counteract this influx, the cell swells and eventually undergoes lysis.
This mechanism of action is bactericidal, meaning it leads to the death of the bacterial cell rather than merely inhibiting its growth. However, the efficacy is most pronounced against actively growing and dividing cells, as non-dividing cells have reduced cell wall synthesis activity.
Major Classes of Cell Wall Synthesis Inhibitors
Over the years, several classes of antibiotics targeting bacterial cell wall synthesis have been identified, each with unique structures and modes of action.
Beta-Lactam Antibiotics
Beta-lactam antibiotics are the largest and most widely used group of cell wall synthesis inhibitors. They share a common four-membered beta-lactam ring, essential for their antimicrobial activity. These agents inhibit PBPs, preventing peptidoglycan cross-linking.
Penicillins
Penicillins are the earliest discovered beta-lactams. They exhibit excellent activity against Gram-positive bacteria and certain Gram-negative species. Examples include penicillin G, amoxicillin, and oxacillin. Their clinical applications range from treating streptococcal infections to syphilis and skin infections.
Cephalosporins
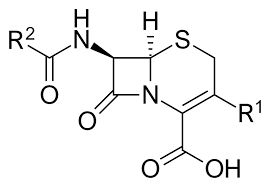
Cephalosporins possess a broader spectrum than penicillins and are divided into generations based on their antibacterial activity and resistance to beta-lactamases. Common examples include ceftriaxone for meningitis and cephalexin for respiratory tract infections.
Carbapenems
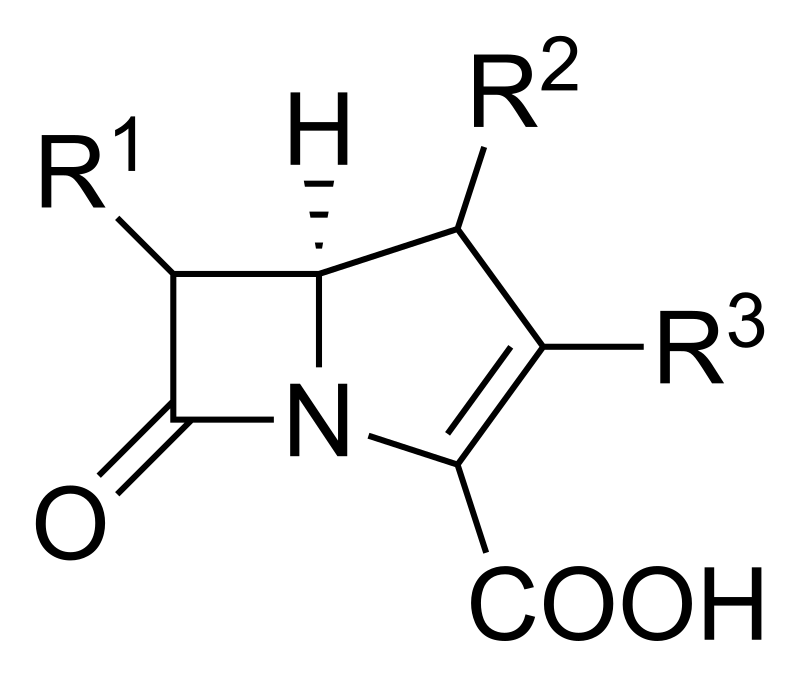
Carbapenems, such as meropenem, have a broad spectrum of activity and are reserved for treating severe or multidrug-resistant infections. They are highly resistant to most beta-lactamases.
Monobactams
Monobactams, like aztreonam, are active mainly against Gram-negative bacteria and exhibit minimal cross-reactivity with other beta-lactams, making them useful in patients with beta-lactam allergies.
Glycopeptide Antibiotics
Glycopeptides inhibit cell wall synthesis by binding to the D-Ala-D-Ala termini of peptidoglycan precursors, preventing cross-linking.
Vancomycin
Vancomycin is highly effective against Gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA). It is commonly used for severe infections like endocarditis and osteomyelitis.
Teicoplanin
Teicoplanin is similar to vancomycin but has a longer half-life, allowing once-daily dosing. It is often used for Gram-positive bacterial infections, especially in patients who cannot tolerate vancomycin.
Fosfomycin
Fosfomycin inhibits an earlier stage of peptidoglycan synthesis by blocking the enzyme MurA, responsible for forming N-acetylmuramic acid. It is mainly used for treating uncomplicated urinary tract infections.
Bacitracin
Bacitracin interferes with the transport of peptidoglycan precursors across the bacterial cell membrane. It is primarily used topically due to nephrotoxicity when administered systemically.
Cycloserine
Cycloserine inhibits the enzymes involved in the synthesis of peptidoglycan precursors. It is reserved for treating multidrug-resistant tuberculosis due to potential neurotoxicity.
Notable Examples and Their Clinical Applications
Certain antibiotics from the classes mentioned earlier are particularly noteworthy because of their proven efficacy in treating specific infections. These agents have become cornerstones in clinical microbiology and infectious disease management.
Amoxicillin and Streptococcal Infections
Amoxicillin, a widely prescribed penicillin derivative, is highly effective against streptococcal species causing pharyngitis, otitis media, and pneumonia. Its broad-spectrum activity, good oral bioavailability, and favorable safety profile make it a preferred choice for community-acquired respiratory tract infections.
Ceftriaxone in Meningitis
Ceftriaxone, a third-generation cephalosporin, penetrates the cerebrospinal fluid efficiently, making it suitable for treating bacterial meningitis caused by Neisseria meningitidis and Streptococcus pneumoniae. Its long half-life allows for once or twice daily dosing, improving patient compliance.
Meropenem in Multi-Drug Resistant Infections
Meropenem is a potent carbapenem with broad-spectrum activity against Gram-positive, Gram-negative, and anaerobic bacteria. It is particularly valuable for treating serious hospital-acquired infections and infections caused by multidrug-resistant organisms, such as extended-spectrum beta-lactamase (ESBL) producers.
Vancomycin in MRSA (Methicillin-Resistant Staphylococcus aureus)
Vancomycin remains the drug of choice for infections caused by MRSA, a major pathogen in hospitals worldwide. It is also used to treat enterococcal infections and Clostridium difficile-associated pseudomembranous colitis (orally).
Fosfomycin in Urinary Tract Infections
Fosfomycin is administered orally in a single-dose regimen for uncomplicated urinary tract infections, particularly those caused by Escherichia coli, including multidrug-resistant strains. Its unique mechanism of action complements other cell wall inhibitors.
Mechanisms of Bacterial Resistance to Cell Wall Synthesis Inhibitors
As with most antimicrobials, the widespread and sometimes inappropriate use of cell wall inhibitors has led to the emergence of resistant bacterial strains. Bacteria employ several strategies to evade the effects of these agents, compromising their clinical utility.
Beta-Lactamase Production
One of the most common resistance mechanisms is the production of beta-lactamase enzymes, which hydrolyze the beta-lactam ring, rendering the antibiotic inactive. Different classes of beta-lactamases, including ESBLs and carbapenemases, target various beta-lactam antibiotics.
Modification of Penicillin-Binding Proteins (PBPs)
Some bacteria, particularly Streptococcus pneumoniae and MRSA, develop resistance by altering the structure of their PBPs. These modified PBPs have reduced affinity for beta-lactam antibiotics, allowing peptidoglycan synthesis to continue in the presence of the drug.
Reduced Permeability in Gram-Negative Bacteria
Gram-negative bacteria possess an outer membrane that limits the penetration of antibiotics into the periplasmic space, where PBPs are located. Alterations in porin proteins can further reduce antibiotic entry, enhancing resistance.
Efflux Pumps and Other Resistance Mechanisms
Certain bacteria use efflux pumps to actively expel antibiotics from the cell, lowering intracellular drug concentrations. Additionally, some species can bypass inhibited enzymes or produce alternative pathways for cell wall synthesis.
Diagnostic Techniques for Detecting Resistance
Timely and accurate identification of antibiotic resistance is crucial for effective infection management and controlling the spread of resistant pathogens.
Antibiotic Susceptibility Testing (AST)
Conventional AST methods, such as disk diffusion, broth microdilution, and E-test, determine the minimum inhibitory concentration (MIC) of an antibiotic against a bacterial isolate. These tests guide clinicians in selecting appropriate therapy.
Molecular Diagnostics (PCR, DNA Sequencing)
Molecular techniques offer rapid and precise detection of resistance genes, such as mecA in MRSA or bla genes encoding beta-lactamases. Polymerase chain reaction (PCR) and DNA sequencing help identify specific mutations and resistance determinants.
Current Challenges in Antimicrobial Therapy
The rise of antibiotic resistance presents significant obstacles to modern medicine. Infections once easily curable with standard antibiotics are now increasingly difficult to treat.
Rising Prevalence of Multi-Drug Resistant Pathogens
The global spread of multidrug-resistant bacteria, including carbapenem-resistant Enterobacteriaceae and vancomycin-resistant Enterococci (VRE), threatens the effectiveness of current antimicrobial agents. These infections are associated with higher morbidity, mortality, and healthcare costs.
Limited Availability of Novel Antibiotics
Despite growing resistance, the development of new antibiotics has slowed considerably. Financial, regulatory, and scientific challenges have contributed to a stagnating antibiotic pipeline, exacerbating the resistance crisis.
New Developments in Inhibitor Research
To address emerging resistance, researchers are developing new agents and therapeutic strategies aimed at restoring the efficacy of existing antibiotics and introducing novel drugs.
Beta-Lactamase Inhibitors (Clavulanic Acid, Avibactam)
Beta-lactamase inhibitors are compounds that bind irreversibly to beta-lactamase enzymes, protecting beta-lactam antibiotics from degradation. Clavulanic acid, combined with amoxicillin, and newer agents like avibactam have expanded treatment options against resistant organisms.
Next-Generation Glycopeptides
Modified glycopeptides, such as dalbavancin and oritavancin, exhibit improved activity against resistant Gram-positive bacteria and possess extended half-lives, allowing for less frequent dosing.
Combination Therapies
Combining different classes of antibiotics or using beta-lactamase inhibitors with beta-lactams enhances efficacy against resistant pathogens and reduces the risk of resistance emergence. Such combinations are increasingly employed in clinical practice.
Significance in Clinical Microbiology and Public Health
Cell wall synthesis inhibitors play a pivotal role in managing bacterial infections and maintaining public health. Their proper use is essential to preserving their effectiveness and preventing the spread of resistant strains.
Importance in Hospital Infection Control
These antibiotics are integral to controlling hospital-acquired infections, especially in intensive care units and post-surgical settings. Infection control measures combined with appropriate antimicrobial stewardship programs help limit resistance development.
Use in Empirical Therapy
In life-threatening infections where immediate treatment is necessary, cell wall inhibitors are often included in empirical therapy due to their broad-spectrum activity and rapid bactericidal effect. Once pathogen identification and susceptibility results are available, therapy is refined accordingly.
Conclusion
Cell wall synthesis inhibitors remain a cornerstone of antimicrobial therapy due to their selective toxicity and potent bactericidal activity. While their historical success has saved countless lives, the growing challenge of antibiotic resistance threatens their future efficacy.
Continuous surveillance for resistance, rational antibiotic use, and investment in novel antimicrobial research are vital to preserving these life-saving drugs. The development of new inhibitors, beta-lactamase inhibitors, and combination therapies offers hope in combating resistant pathogens and safeguarding global public health.
Frequently Asked Questions (FAQ)
Why is the bacterial cell wall an effective antibiotic target?
Because human cells don’t have a cell wall, so antibiotics that attack the bacterial cell wall can kill bacteria without harming our own cells. It’s a unique, essential structure for bacterial survival, making it a safe and effective target.
What is the difference between bactericidal and bacteriostatic drugs?
Bactericidal drugs kill bacteria outright, while bacteriostatic drugs just stop them from growing and multiplying letting the body’s immune system finish the job.
How do beta-lactamase inhibitors work?
They block the enzymes (called beta-lactamases) made by some bacteria to destroy beta-lactam antibiotics. By disabling these enzymes, the inhibitors protect the antibiotics and help them stay effective against bacteria.
Related Contents
Cell Proliferation – Definition, Assay, Differentiation, Diseases