Introduction
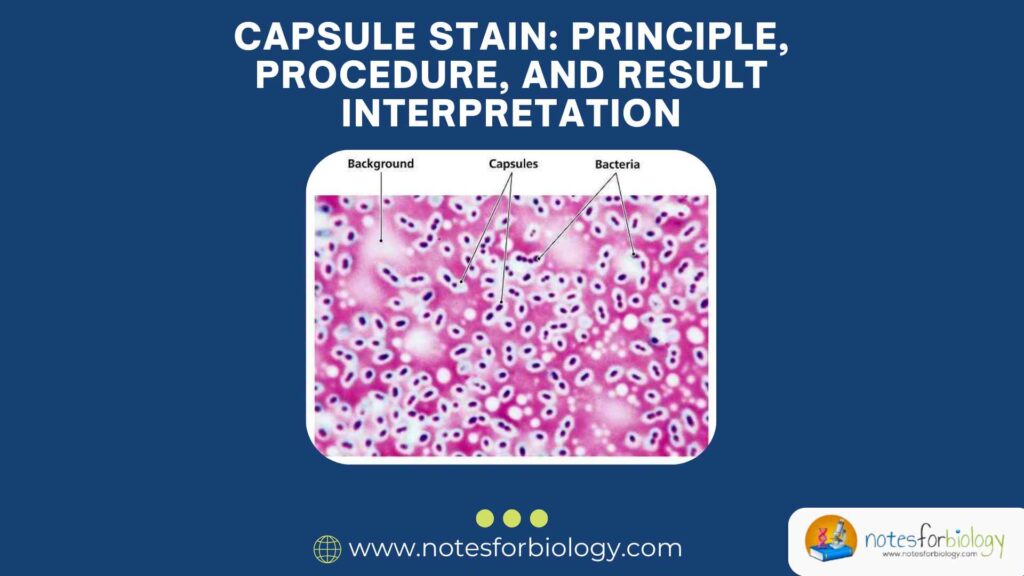
The capsule stain is a specialized microbiological technique used to visualize bacterial capsules—gelatinous, protective layers surrounding some bacterial cells. These capsules play crucial roles in virulence, immune evasion, and biofilm formation. Unlike routine staining methods, capsule staining requires special approaches because capsules are water-soluble and non-ionic, making them resistant to conventional stains. This guide explains the principle, step-by-step procedure, and interpretation of capsule staining in detail.
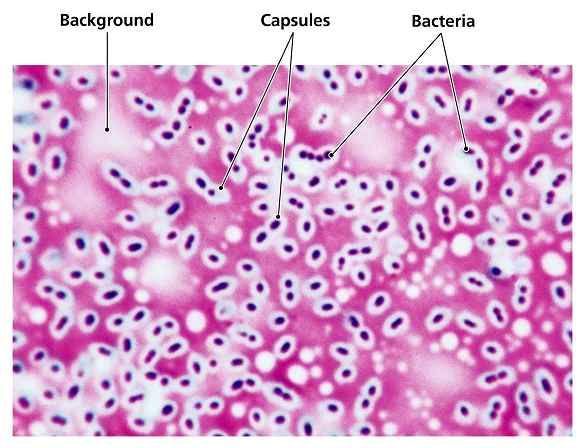
Table of Contents
1. Principle of Capsule Staining
Bacterial capsules consist mainly of polysaccharides (or sometimes polypeptides) that repel most standard stains. Since capsules are non-ionic and hydrophilic, they do not take up ordinary dyes. Instead, capsule staining relies on two key principles:
Negative Staining (Background Staining)
- An acidic stain (like India ink or Congo red) colors the background but not the capsule.
- This creates a dark field around the cell, making the capsule appear as a clear halo.
Positive Staining (Cell Staining)
- A simple stain (like crystal violet or safranin) colors the bacterial cell but not the capsule.
- The capsule remains unstained, appearing as a translucent zone between the cell and the background.
Why Capsules Resist Staining:
- Capsules are water-soluble and wash away during routine staining.
- They lack electrical charge, preventing dye binding.
- Heat fixation (used in Gram staining) can destroy or shrink capsules.
2. Types of Capsule Staining Methods
Two main techniques are used:
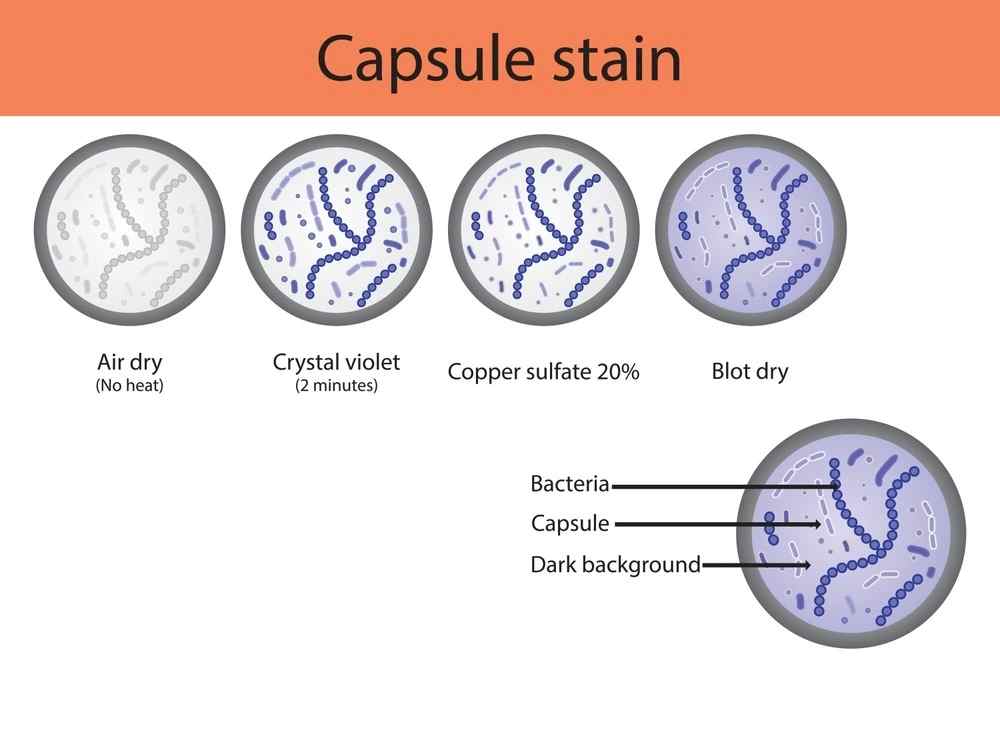
A. Anthony’s Stain Method
- Uses crystal violet (stains the cell) and copper sulfate (washes excess dye while preserving the capsule).
- Best for Gram-positive encapsulated bacteria (e.g., Streptococcus pneumoniae).
B. Maneval’s Stain (India Ink Method)
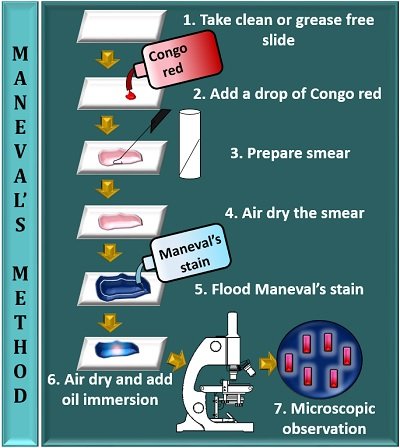
- Uses India ink (dark background) and acidic stain (e.g., Congo red).
- Ideal for Gram-negative encapsulated bacteria (e.g., Klebsiella pneumoniae).
3. Step-by-Step Procedure (Maneval’s India Ink Method)
Materials Required:
- Bacterial culture (e.g., Klebsiella or Bacillus spp.)
- Microscope slides
- India ink (or Congo red)
- Acidic stain (e.g., 1% acetic acid)
- Basic stain (e.g., safranin or crystal violet)
- Bunsen burner (for heat fixation, if needed)
- Microscope (1000x magnification, oil immersion)
Procedure:
Prepare a Thin Smear:
- Place a small drop of India ink on a clean slide.
- Mix a loopful of bacterial culture into the ink.
- Spread thinly using another slide (like a blood smear).
Air Dry (Do Not Heat Fix!)
Heat can distort the capsule. Let it dry naturally.
Apply Acidic Stain (Optional):
Flood the slide with 1% acetic acid for 1 minute (enhances contrast).
Rinse Gently:
Use distilled water to wash off excess stain.
Counterstain (Optional):
Apply safranin (or crystal violet) for 30 seconds to stain the cell.
Rinse & Dry:
Wash gently and air dry.
Microscopic Examination:
Use oil immersion (1000x magnification).
4. Expected Results & Interpretation
| Observation | Interpretation |
|---|---|
| Clear halo around cells | Capsule present (unstained zone between cell and dark background) |
| Dark background | Negative stain (India ink) working correctly |
| Pink/red cells | Safranin-stained bacterial cells |
| No halo | No capsule present (or staining error) |
Example:
- Klebsiella pneumoniae → Clear capsule halo around pink rods.
- Streptococcus pneumoniae → Translucent zone around purple cocci.
5. Common Errors & Troubleshooting
| Problem | Possible Cause | Solution |
|---|---|---|
| No capsule visible | Over-rinsing or heat fixation | Avoid heat; rinse gently |
| Faint background | Too little India ink | Increase ink concentration |
| Cells not visible | Weak counterstain | Extend safranin staining time |
| Capsule looks distorted | Smear too thick | Prepare a thinner smear |
6. Applications of Capsule Staining
Medical Microbiology:
- Identifies pathogenic bacteria (S. pneumoniae, K. pneumoniae).
- Helps diagnose meningitis, pneumonia.
Research:
- Studies bacterial virulence factors.
- Evaluates vaccine development (capsule-based vaccines).
Industrial & Environmental:
- Detects biofilm-forming bacteria in water systems.
7. Limitations
- Not all bacteria have capsules.
- False negatives if capsules are too thin.
- Requires practice to distinguish capsules from artifacts.
Conclusion
Capsule staining is a vital technique for identifying encapsulated bacteria, crucial for medical diagnostics and research. Unlike Gram staining, it requires negative staining to highlight the capsule’s translucent layer. Proper technique (avoiding heat fixation, gentle rinsing) ensures accurate results. Mastering this method helps microbiologists study bacterial virulence and develop targeted treatments.
FREQUENTLY ASKED QUESTIONS
What is capsule staining?
Capsule staining is a special microbiological technique used to visualize the gelatinous capsule layer surrounding certain bacteria. Unlike routine stains, it uses a combination of negative and positive staining to make these water-soluble structures visible under a microscope.
Why can’t we use Gram staining for capsules?
Capsules are water-soluble and wash away during Gram staining.
They lack charge, so most dyes don’t bind to them.
Heat fixation (used in Gram staining) destroys capsules.
Why is India ink used?
India ink provides a dark background, creating contrast to see the capsule as a translucent zone around the cell.
Related Articles