Bial’s Test is a biochemical test used to determine whether pentoses, or sugars with five carbons, are present in a sample. When separating pentoses from hexoses (six-carbon sugars) and other carbohydrates, this test is especially helpful. A chemical test called Bial’s Test is used to identify pentose carbohydrates. The test is based on the production of a green hue by the reaction between pentoses and orcinol in the presence of strong hydrochloric acid.
Pentose and compounds generated from pentose (pentosans) can be distinguished from other carbohydrates using this test. Pentoses are simple sugars composed primarily of five carbon units. The most prevalent forms of pentose and its derivatives include hemicellulose, xylose, ribose, arabinose, and deoxyribonucleic acid (DNA) and ribonucleic acid (RNA).
Table of Contents
Bial’s Test

Principle
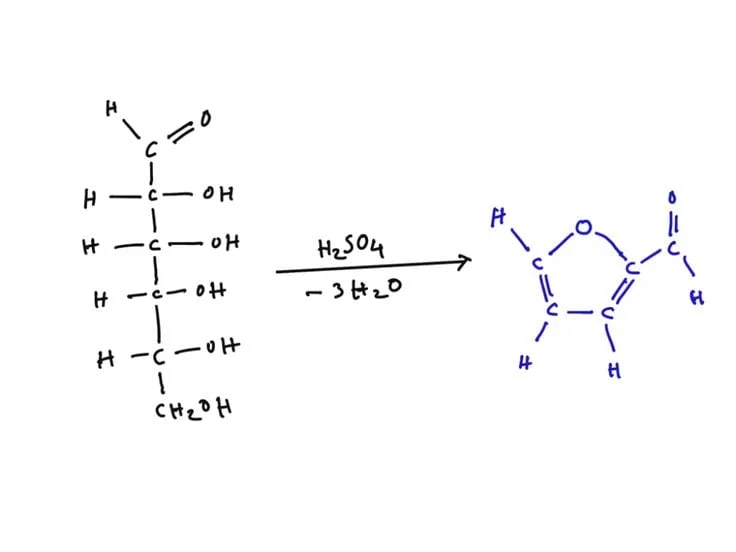
The dehydration of pentoses by strong hydrochloric acid to produce furfural is the basis for the this principle. After that, the furfural combines with ferric chloride (FeCl₃) and orcinol to form a complex that is tinted blue-green. If hexoses are present, they dehydrate to produce hydroxymethylfurfural, which reacts with orcinol to provide a distinct color.
The purpose of Bial’s test is to identify pentose and compounds produced from pentose in analytes. Additionally, it facilitates the separation of pentose monosaccharides from other types of carbohydrates.
This test is based on the idea that a blue-green complex is produced when reagent combines with a sample that contains pentose or pentose derivatives. In this case, pentose derivatives decompose back to pentose. Subsequently, furfural is formed when concentrated hydrochloric acid (HCl) in Bial’s reagent dehydrates pentose.
Pentose/pentose derived compounds + H+ (from concentrated HCl) → Furfural
Furfural + FeCl3 + Orcinol → Blue-green complex
Procedure
Preparation of Reagents
Mix 0.5 grams of orcinol with 100 milliliters of strong hydrochloric acid (HCl) to create Bial’s Reagent. Drops of a 10% ferric chloride (FeCl₃) solution should be added.
Test Procedure
- Place a test tube with 1-2 mL of the sample solution.
- Transfer 2–3 milliliters of Bial’s reagent into the test tube.
- For around five minutes, slowly warm the mixture in a bath of boiling water.
- See how the hue changes.
Result
- Positive Outcome: The presence of pentoses is indicated by a green to blue-green tint.
- Negative Outcome: The absence of pentoses is indicated by a yellow to brown tint or by no discernible color change.
| Tested substances | Observation | Interpretation |
| Positive control (xylose-filled test tube) | Light green to blue-green in color. | Positive Bial’s test |
| Negative control: a water-filled test tube | No change in color | Negative Bial’s test |
| Test (tube with samples) | The hue turns blue-green The hue turns dirty brown | ositive Bial’s test (Presence of pentose or pentose derivatives) 2. Negative Bial’s test (Absence of pentose or pentose derivatives) |
Uses
- Pentose Detection: In biological samples, pentoses like ribose and xylose are mainly found using Bial’s Test.
- Nucleic Acid Testing: As ribose is a component of RNA, nucleic acid testing can be used to determine whether RNA is present in a sample.
- Differentiating Carbohydrates: In carbohydrate analysis, it aids in separating pentoses from hexoses.
Conclusion
Bial’s Test is a vital tool in biochemical laboratories, especially in the areas of genetics and molecular biology for nucleic acid analysis, as it provides a simple and accurate way to identify pentose sugars in a variety of materials.A useful biochemical test for identifying pentose sugars and nucleic acids in a variety of samples is the Bial’s Test. The test is based on the creation of a colorful complex between furfural and orcinol, which is created when pentoses are dehydrated in an acidic environment. Pentoses like ribose or xylose are confirmed to be present in a positive result, denoted by a green to blue-green color; in contrast, the absence of considerable color change shows their absence.
Frequently Asked Question(FAQ)
Define Bial’s Test ?
Bial’s Test is a biochemical test used to determine whether pentoses, or sugars with five carbons, are present in a sample. When separating pentoses from hexoses (six-carbon sugars) and other carbohydrates, this test is especially helpful.
What is the principle of Bial’s test?
The underlying idea of this test is that pentosans hydrolyze into pentoses when they are hydrolyzed. Pentoses are further dehydrated to produce furfural, which condenses with orcinol to form a precipitate that is blue-green in color.
What are the expected results of Bial’s test?
The expected outcome of the test is the formation of a blue-green color, indicating the presence of glucose .
What is positive for Bial’s test?
Positive Bial’s test: the development of a blue hue (ribose sugar, for example). Any other color formation indicates a negative test, according to Bial’s negative test. Generally, hexose sugar (glucose, fructose) produces products that are green, red, or brown in hue.
Related Article