ATP Synthase is an essential enzyme present in the membranes of many bacteria, chloroplasts, and mitochondria. It converts inorganic phosphate (Pi) and adenosine diphosphate (ADP) into adenosine triphosphate (ATP), the main energy source in cells. Protons (H+) passing through a membrane and producing an electrochemical gradient are what propel this process. The energy required for several cellular processes is produced when ATP synthase transforms the energy from this proton gradient into chemical energy stored in ATP.
Table of Contents
Here is a detailed analysis of its structure, mechanism, and significance:
Structure
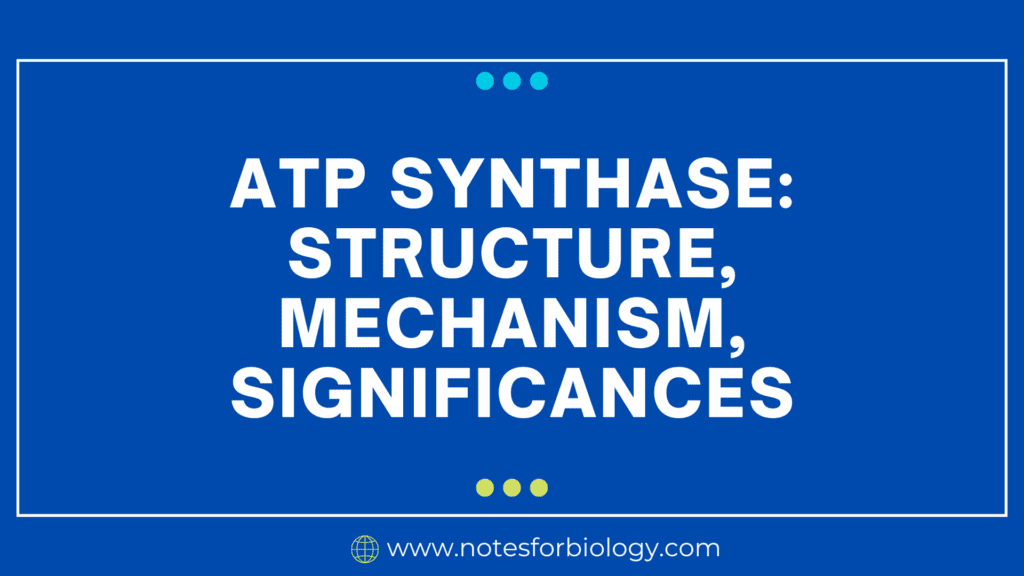
ATP synthase is a big, intricate enzyme with several subunits that are split into two primary parts:
1. F0 Component
- Position: Integrated within the inner membrane of the mitochondria (or prokaryotic plasma membrane and chloroplast thylakoid membrane).
- Structure: Consists of many subunits that together form a channel that allows protons (H+) to pass through. The principal subunits consist of:
- a subunit: Has proton translocation channels.
- b subunit: Joins the F0 and F1 parts together.
- c ring: Consists of 10–14 c subunits, which spin to allow protons to flow through.
2. F1 Component:
- Location: Located within the mitochondrial matrix (stroma of chloroplasts and cytoplasm of prokaryotes).
- Structure: Consists of five distinct subunits (α, β, γ, δ, and ε) organized in a complicated configuration:
- α3β3 hexamer: ATP production takes place within a hexameric ring formed by the alternation of α and β subunits.
- γ subunit: Functions as a rotor by extending into the middle of the α3β3 hexamer.
- Subunits δ and ε: Provide structural organization and stabilize the complex.
Mechanism
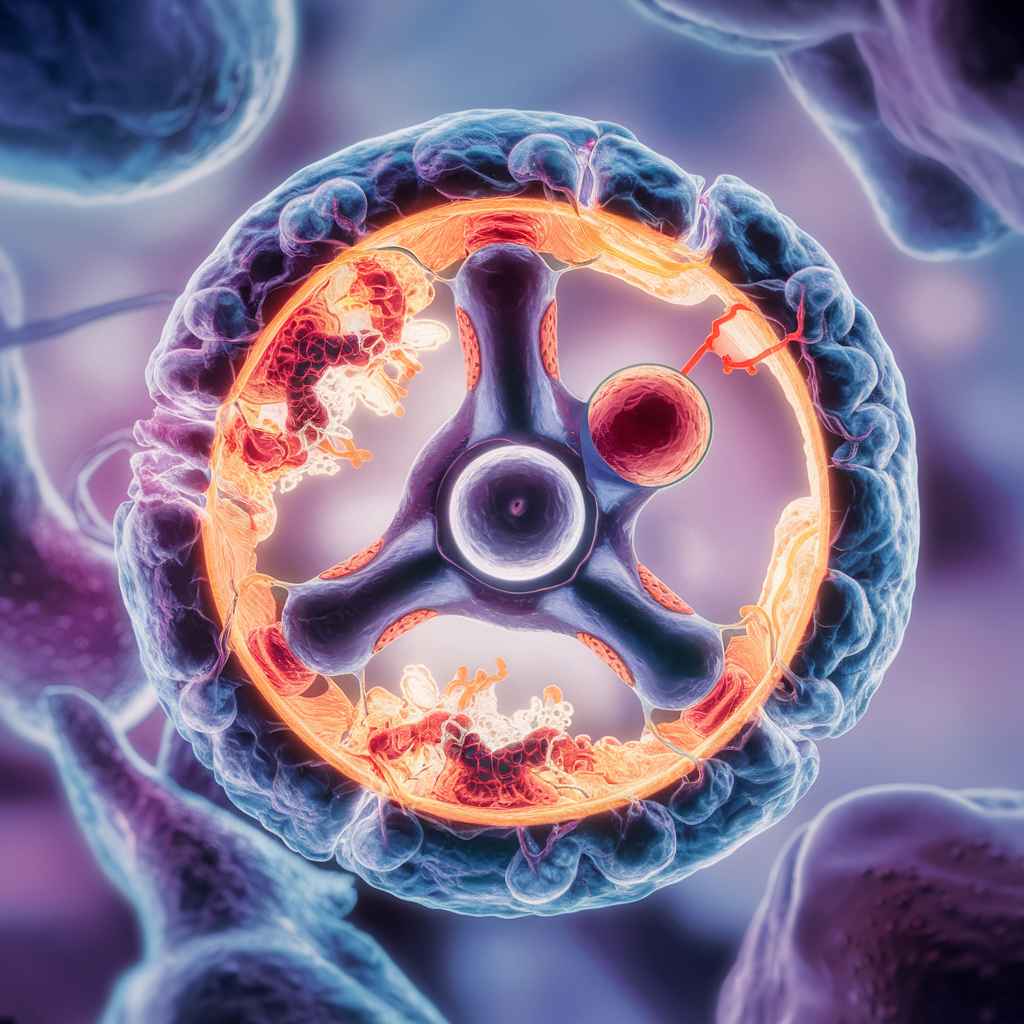
By linking proton translocation and ATP production, ATP synthase functions via a chemiosmotic mechanism known as the rotary mechanism:
1. Proton Translocation
- The electron transport chain pumps protons (H+) across the mitochondrial membrane (or its equivalent), resulting in an electrochemical gradient and the proton motive force.
- Protons return through the F0 component of ATP synthase to the mitochondrial matrix (or cytoplasm/stroma).
2. Rotary Catalysis
- The rotation of the c ring is caused by protons passing through the F0 component.
- The γ subunit receives this rotation and turns inside the F1 component’s α3β3 hexamer.
- The β subunit’s rotation causes the β subunits to undergo conformational changes, causing them to alternate between three states: loose (L), tight (T), and open (O).
- L state: Pi binds to ADP.
- T state: ADP and Pi are used to create ATP.
- O state: the release of ATP.
Significance
1. Energy Production
- ATP synthase is the main enzyme that produces ATP, the universal energy unit of cells, during photophosphorylation in chloroplasts and oxidative phosphorylation in mitochondria.
- It permits cells to transform electrochemical gradient energy into chemical energy that may be used by the cell.
2. Metabolism in Cells
- A variety of cellular functions, such as biosynthesis, transport, and mechanical labor, are powered by ATP synthase-produced ATP.
- It is necessary for many other physiological processes, including the contraction of muscles and the transmission of nerve impulses.
3. Conservation through Evolution
Since ATP synthase is extremely conserved in many species, it is clear that life depends on it. Because of its comparable structure and function in eukaryotes, bacteria, and archaea, it has been shown to be important in evolution.
4. Medical Relevance
- ATP synthase plays a vital role in maintaining cellular health since deficiencies can result in serious metabolic abnormalities and mitochondrial illnesses.
- Treatments for illnesses involving dysfunctions of energy metabolism can be improved by knowledge of the role and regulation of ATP synthase.
In Addition: The enzyme ATP synthase plays a crucial role in the synthesis of cellular energy by converting adenosine diphosphate (ADP) and inorganic phosphate (Pi) into adenosine triphosphate (ATP). From a structural perspective, it consists of two primary parts: the F0 part, which is enmeshed in the inner mitochondrial membrane (or a similar membrane), and the F1 part, which extends into the mitochondrial matrix (or the cytoplasm/stroma). A proton channel made up of subunits a, b, and a c-ring is formed by the F0 component, while a hexameric ring structure made up of subunits α, β, γ, δ, and ε makes up the F1 component. By linking proton translocation and ATP generation, ATP synthase functions mechanistically via a chemiosmotic process.
Frequently Asked Question(FAQ)
What is ATP synthase and where is it located?
The enzyme ATP synthase is present in the membranes of many bacteria, chloroplasts, and mitochondria. It converts inorganic phosphate (Pi) and adenosine diphosphate (ADP) into adenosine triphosphate (ATP).
What is the structure and mechanism of ATP synthase?
The two main parts of ATP synthase are the F0 component, F1 component. From a mechanistic perspective, ATP synthase combines proton translocation and ATP production through a chemiosmotic mechanism. Protons go through the F0 component, causing the c-ring and γ subunit to rotate.
Related Articles