Antimicrobial Susceptibility Testing (AST) is laboratory technique used to find out how sensitive different antimicrobial drugs are to different types of bacteria. The choice of the most suitable antibiotic therapy for infections is aided by this testing. A crucial element of contemporary clinical microbiology and infectious disease treatment, Antimicrobial Susceptibility Testing supports both the worldwide fight against antibiotic resistance and efficient patient care.
Instead of measuring antimicrobial activity in vivo (against germs in a patient), susceptibility testing assess it in vitro (against bacteria in a laboratory). Therefore, it cannot be expected that an antibacterial that eradicates or stops an organism from proliferating in vitro would work as a remedy.
Table of Contents
Antimicrobial Susceptibility Testing
Types of Antimicrobial Susceptibility Testing
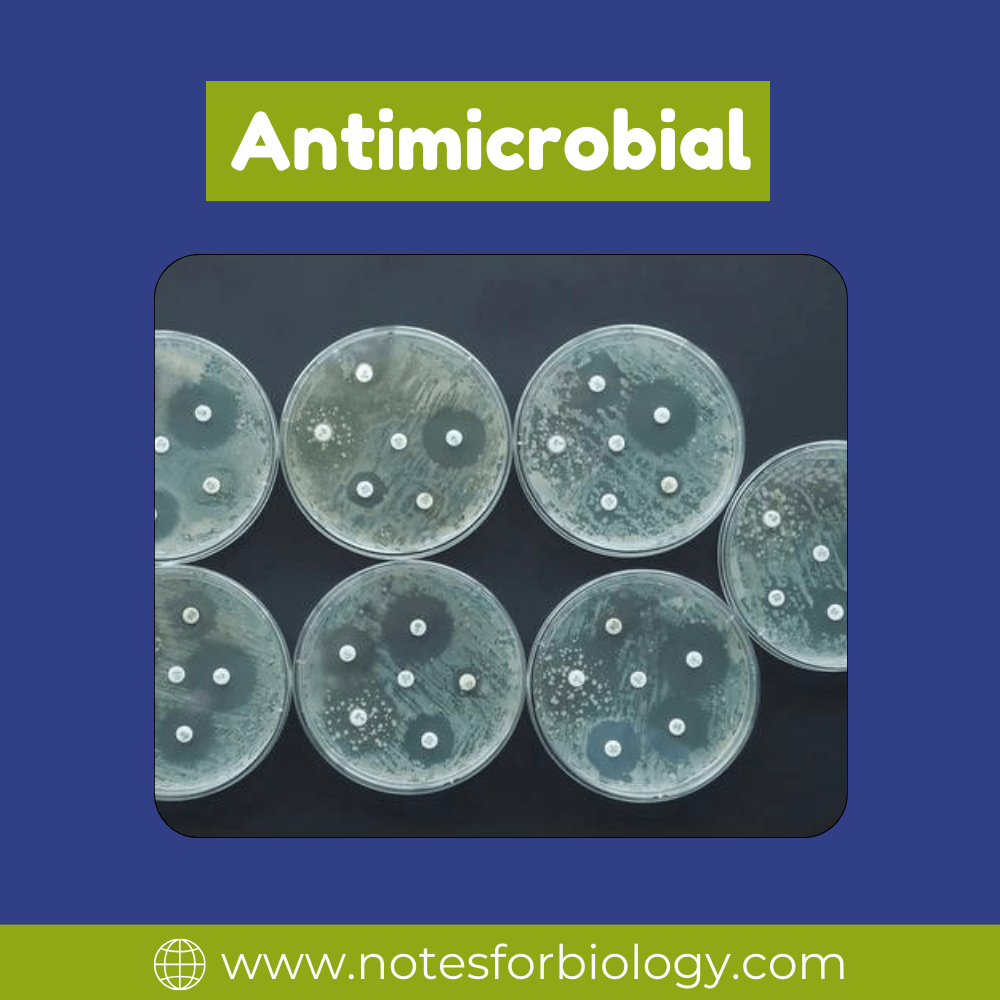
Disk Diffusion Method (Kirby-Bauer Test)
Method: Antibiotic-impregnated disks are placed on the surface of an agar plate after bacterial isolates have been spread out on it. After that, the plates are incubated to promote bacterial growth and antibiotic diffusion.
Findings: Measurements are made of the inhibitory zones surrounding the disks. The bacteria’s antibiotic susceptibility is correlated with the size of these zones.
Benefits: Easy to use and reasonably priced.
extensively utilized and standardized by CLSI (Clinical and Laboratory Standards Institute) and similar organizations.
Limitations: Results are more qualitative than quantitative.
In agar, some antibiotics may not spread evenly.
Broth Dilution Method (Microdilution and Macrodilution)
Method: Bacterial isolates are cultivated in broth containing progressively diluted antibiotics. The Minimum Inhibitory Concentration (MIC) of the antibiotic is the lowest concentration at which growth is observably inhibited.
Findings: MIC values are discovered.
Benefits: Quantitative outcomes with precise MIC values.
able to test several antibiotics at once in a microdilution format.
Limitations: Needs specialized equipment and is labor-intensive.
costlier than that of disk diffusion.
Possibility of human error when making dilutions
E-test (Epsilometer Test)
Method: An agar plate that has been infected with the bacteria is covered with a strip that has been impregnated with a gradient of antibiotic. An oval zone of inhibition forms during incubation, and the scale on the strip can be used to determine the MIC directly.
Findings: Direct MIC values are the results.
Benefits: Easy to execute and comprehend.
gives exact MIC values.
Drawbacks: Costlier than disk diffusion.
Some antibiotics have limited supply of strips.
Automated Systems (e.g., VITEK, BD Phoenix)
Method: Susceptibility testing is carried out by automated devices utilizing pre-made antibiotic panels. Bacterial growth is tracked, and complex algorithms are used to calculate MICs.
Findings: MIC values and susceptibility profiles are the outcomes.
Benefits: Quick turnaround times and high throughput.
labor costs are lower and human error is possible.
Limitations: Exorbitant startup and ongoing expenses.
requirements for calibration and upkeep.
restricted freedom to try novel medications or non-standard antibiotics.
Molecular Methods (e.g., PCR, Next-Generation Sequencing)
Methodology: These techniques use bacterial DNA to directly identify genetic markers of resistance.
Findings: Specific resistance genes present or absent.
Benefits: Quick identification of resistance genes.
beneficial for creatures that are challenging to cultivate.
Limitations: No MIC values are provided.
might not identify every resistance mechanism.
Expensive and requiring specific tools and knowledge.
Limitations of Antimicrobial Susceptibility Testing
Technical Limitations
- Results may vary depending on the test settings (e.g., medium composition, incubation period).
- testing certain picky or slowly growing organisms might be challenging.
- Some techniques need specific tools and are labor-intensive.
Clinical Limitations
- Because of things like drug pharmacokinetics and the infection site, in vitro outcomes do not necessarily translate to in vivo efficacy.
- Initial susceptibility testing may not be able to predict the emergence of resistance during therapy.
Economic Limitations
- Labor, supplies, and equipment costs can be significant, especially for automated and molecular techniques.
- Restricted accessibility of specific assessments in environments with limited resources
Interpretative Limitations
- The MIC thresholds that separate susceptible strains from resistant strains are known as breakpoints for susceptibility, and they might fluctuate over time and between different organizations.
- Multi-infections or polymicrobial cultures can make interpreting the results more difficult.
Notwithstanding these drawbacks, Antimicrobial Susceptibility Testing is still an essential instrument for managing infectious diseases and clinical microbiology, aiding in the direction of efficient care and the fight against antibiotic resistance. It is strictly forbidden to conduct Antimicrobial Susceptibility Testing on commensal organisms or pollutants as this could mislead the clinician, lead to the patient receiving unnecessary and ineffective antimicrobial therapy, result in potential side effects, and cause the patient to become resistant to other potentially pathogenic organisms.
Frequently Asked Question (FAQ)
What is Antimicrobial Susceptibility Testing ?
Antimicrobial Susceptibility Testing (AST) is laboratory technique used to find out how sensitive different antimicrobial drugs are to different types of bacteria.
State two types of Antimicrobial Susceptibility Testing?
The two types of Antimicrobial Susceptibility Testing is E-test (Epsilometer Test), Disk Diffusion Method (Kirby-Bauer Test).
What are the two limitations of Antimicrobial Susceptibility Testing?
Results may vary depending on the test settings (e.g., medium composition, incubation period).
Labor, supplies, and equipment costs can be significant, especially for automated and molecular techniques.
What are the examples of Molecular Methods?
PCR, Next-Generation Sequencing
Related Article