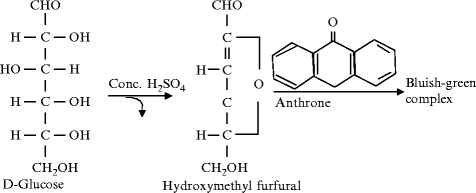
Another broad test for all carbs is the Principle-Anthrone test. Carbohydrates in this test also get dehydrated when they react with concentrated H2SO4 to generate furfural. When this furfural combines with anthrone, a blue green complex is produced.
Definition
A colorimetric assay called the Anthrone Test is used to measure the amount of carbohydrates in a sample, more precisely the total amount of sugars and polysaccharides. In clinical and biochemical labs, it’s a frequently used, straightforward test.
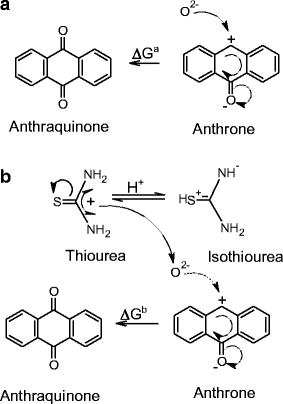
Principle
The basis of the Anthrone Test is the blue-green complex that is created when carbohydrates and anthrone reagent combine in an acidic environment. This reaction happens when sulfuric acid is present and dehydrates carbohydrates into furfural derivatives, which subsequently react with anthrone to produce the colorful complex. The amount of carbs present in the sample directly correlates with the color’s intensity.
Procedure
1. Setting Up the Reagents
Anthrone Reagent: 100 mL of concentrated sulfuric acid (H3SO4) should be used to dissolve 0.2 g of anthrone. Make this solution right away and store it in the refrigerator.
2. Sample Preparation
Take 1 milliliter (mL) of the carbohydrate-containing sample solution.
To make sure the carbohydrate concentration is within the assay’s detection range, dilute the sample if needed.
3. Reaction Setup
In a test tube, add 4 milliliters of the recently made anthrone reagent to the sample solution.
Completely blend by rotating the test tube.
4. Heating
For ten minutes, the mixture is heated in a bath of boiling water. To preserve accuracy, make sure that every sample is timed in the same way.
Cool the tubes to room temperature after heating, ideally in a bath of cold water.
5. Measurement
Using a spectrophotometer, find the blue-green solution’s absorbance at 620 nm.
Create a blank by processing it in the same way as the samples using anthrone reagent and distilled water.
6. Standard Curve
Using known concentrations of a carbohydrate standard (such as glucose or sucrose), create a standard curve.
To build the standard curve, plot absorbance vs concentration.
Result
By comparing the absorbance of the unknown sample to the standard curve, one may calculate the concentration of carbohydrates present in the sample.
larger absorbance corresponds to a larger concentration of carbs since the absorbance value is related to the content of carbohydrates.
Uses
1. Research on Biochemistry
total carbohydrate content measured in biological samples, including blood, urine, plant extracts, and dietary items.
measurement of the polysaccharides, starch, and glycogen present in tissues and cells.
2. Clinical Uses
tracking the amounts of carbohydrates under different physiological and pathological circumstances.
A diagnostic tool for metabolic diseases related to the metabolism of carbohydrates.
3. Agriculture and the Food Sectors
figuring out a food product’s carbohydrate amount for nutritional labeling.
Analyzing the carbohydrate content of agricultural goods allows for quality monitoring.
4. Science of the Environment
examination of soil and water sample organic carbon content.
Frequently Asked Question
1. What is the principle of anthrone test?
The concentrated acid in the Anthrone reagent first hydrolyzes the carbohydrate into component monosaccharide whether it is present as free carbohydrate as poly- or monosaccharide or bound as in a glycoprotein or glycolipid.
2. What is the use of anthrone?
Anthrone is an aromatic ketone that is tricyclic. It is employed in the colorimetric detection of carbohydrates as well as a standard cellulose test. Pharmacy uses derivatives of anthrone as a laxative. They decrease the absorption of water and increase intestinal motility.
3. What is the color of the reagent in anthrone?
Anthrone, also known as 9(10H)-Anthracenone, is a planar tricyclic aromatic ketone. In acidic conditions, anthrone reacts with carbohydrate to yield a blue-green color
Related Article