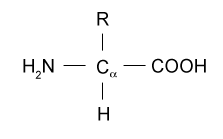
As the building blocks of proteins, amino acids are essential to many biological processes. From a structural standpoint,it is usually made up of an amino group, a variable group, a carbon atom, a hydrogen atom, and a carboxyl group.
Table of Contents
It can be divided into four groups based on the variable group: nonpolar, polar, negatively charged, and positively charged.
The following provides a thorough summary of their characteristics, organization, categorization, and roles:
Properties of Amino Acids
Physical Properties
- It is crystalline solids without color.
- Every amino acid has a high melting point that exceeds 200 degrees.
- Their solubility is as follows: they dissolve easily in methanol, ethanol, and propanol, but only very weakly in alcohol. The pH of the solvent and the R-group of amino acids are key factors in solubility.
- They decompose at high temperatures.
- Except for glycine, every amino acid has optical activity.
- Formation of peptide bonds: Peptide bonds involving the amino and carboxylate groups of amino acids allow them to bind together. -CO-NH-linkage is the result of a covalent connection developing between the alpha-amino group of one aminoacid and the alpha-carboxyl group of another. Planar, partly ionic peptide linkages are seen in them.
Chemical Properties
1. Zwitterionic Property
A molecule having functional groups—at least one with a positive electrical charge and one with a negative charge—is called a zwitterion. The molecule as a whole has no net charge. They are most well-known zwitterions. They have two types of groups: an acidic carboxylic group and a basic amine group. Due to its stronger base, the -NH2 group takes up H+ from the -COOH group, leaving behind a zwitterion. The typical form of acids in the solution is the (neutral) zwitterion.
2. Amphoteric Property
Since they have two amine and carboxylic groups, they amphoteric, meaning they can function as bases or acids.
3. Sanger’s reagent reaction
In a mildly alkaline media at low temperatures, Sanger’s reagent (1-fluoro-2, 4-dinitrobenzene) combines with a free amino group in the peptide chain.
4. Ninhydrin test
The presence of α-amino acids is shown by the production of a violet color when Ninhydrin solution is heated and added to a protein solution.
5. Xanthoproteic test
Tyrosine, tryptophan, and phenylalanine are examples of aromatic aminoacids that can be found in protein solutions using the xanthoproteic test. Nitric acid reacts with benzoid radicals in the aminoacid chain to produce nitration, which gives the solution its yellow hue.
Structure of Amino Acids
- The building components of proteins are amino acids.
- A NH₂ amine group is present in every amino acid.
- a COOH (carboxyl) group.
- A carbon with alpha (α) bonding.
- A distinct side chain, or R-group.
Classifications of Amino Acids
Based on the characteristics of their side chains, or R groups, they are categorized as follows:
- Nonpolar Amino Acids (Hydrophobic): phenylalanine, proline, valine, leucine, isoleucine, methionine, and tryptophan are a few examples.
- Examples of polar (hydrophilic) uncharged amino acids are glutamine, serine, threonine, cysteine, tyrosine, and asparagine.
- Polar Amino Acids that Are Hydrophilic:
1. Histidine, arginine, and lysine are positively charged (basic).
2. Glutamic acid and aspartic acid are negatively charged (acidic).
Functions of Amino Acids
- Protein Synthesis: The building blocks of proteins, which are necessary for the structure, operation, and control of the body’s tissues and organs, are aminoacids.
- Amino acids near the active site of many proteins, which act as catalysts for biological events, are essential to the activity of the enzyme.
- Signaling Molecules: Certain amino have neurotransmitter-like properties or act as neurotransmitter precursors. One important neurotransmitter in the brain is glutamate, for instance.
- They are involved in a number of metabolic processes, such as the creation of hormones and other chemicals.
- Energy Source: It can be used as an energy source when fasting or engaging in vigorous exercise.
Specific Examples
- The most basic amino acid is glycine, which has a single hydrogen as its R group. It participates in the production of purines and heme and is not chiral.
- Cysteine: Has a thiol group that can join with other molecules to form disulfide bonds, which helps proteins form their tertiary structure.
- The aromatic amino acid phenylalanine is a building block of dopamine, epinephrine, norepinephrine, and tyrosine.
- The most prevalent free amino acid in the body, glutamine serves as both a fuel source for rapidly dividing cells and an essential component in the transfer of nitrogen.
Frequently Asked Question (FAQ)
Why is this acid important ?
As the building blocks of proteins, amino acids are essential to many biological processes.
How many groups can it be divided into ?
It can be divided into four groups based on the variable group: nonpolar, polar, negatively charged, and positively charged.
How many types are they categorized into ?
Based on the characteristics of their side chains, or R groups, they are categorized as follows: Nonpolar(Hydrophobic), polar (hydrophilic) uncharged aminoacids.
Related Article