Alcohol fermentation, or ethanol fermentation, is a biological process that converts carbohydrates like glucose, fructose, and sucrose into cellular energy while producing ethanol and carbon dioxide as byproducts. This process is primarily carried out by yeast and certain types of bacteria.
Table of Contents
What is Ethanol Fermentation?
Ethanol fermentation is the conversion of carbohydrates into ethanol and CO2 by yeast or other microbes. This fermentation technique is widely employed in the manufacturing of alcoholic drinks such as beer and wine.
Steps of Alcohol fermentation (Ethanol)
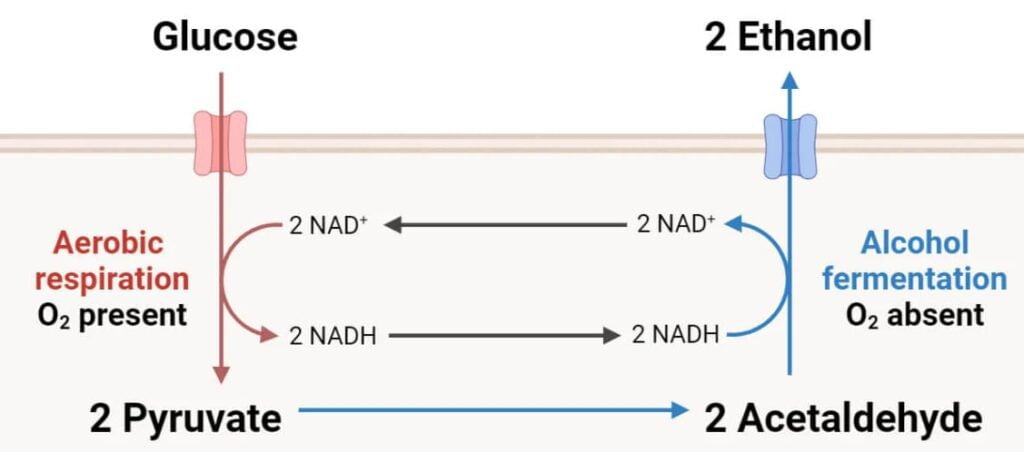
Glycolysis
Location: The cell’s cytoplasm.
Description: During the first stage of fermentation, known as glycolysis, a single glucose molecule (C6H12O6) is split into two pyruvate molecules (CH3COCOO−). In this process, NAD+ is converted to NADH and a tiny quantity of energy is produced in the form of ATP.
Chemical Equation: C6H12O6→2C3H4O3+2ATP+2NADH
Decarboxylation of pyruvate to acetaldehyde
Location: The cell’s cytoplasm.
Description: In the absence of oxygen, pyruvate is transformed into acetaldehyde and CO2. The pyruvate decarboxylase enzyme catalyzes this process.
Chemical Equation: 2C3H4O3→2CH3CHO+2CO2
Reduction of Acetaldehyde to Ethanol
Location: The cell’s cytoplasm.
Description: Acetaldehyde is then converted to ethanol in a process catalyzed by the enzyme alcohol dehydrogenase. During this process, NADH is oxidized back into NAD+, which is required for glycolysis to continue.
Chemical Equation: 2CH3CHO+2NADH+2H+→2CH3CH2OH+2NAD+
Overall Chemical Equation for Ethanol Fermentation
Combining these steps, the overall chemical reaction for ethanol fermentation can be summarized as follows: C6H12O6→2CH3CH2OH+2CO2+ATP
Importance of Each Step
Glycolysis: This phase initiates the breakdown of glucose and produces ATP and NADH, laying the groundwork for fermentation.
Decarboxylation: Reduces pyruvate to a smaller molecule (acetaldehyde) and produces CO2, which is required for the following step.
Reduction: Creates ethanol and regenerates NAD+, allowing glycolysis to proceed and make additional ATP in the absence of oxygen.
Uses of Alcohol Fermentation (Ethanol)
Alcoholic beverages: Alcohol fermentation (Ethanol) is the process used to make alcoholic beverages including beer, wine, and spirits. Different yeast strains and fermentation conditions are utilized to create diverse sorts of alcoholic beverages with varied flavors and alcohol levels.
Food Industry: Fermentation is utilized in the manufacturing of many food products, including bread. Yeast fermentation generates carbon dioxide, causing dough to rise.
Pharmaceuticals: Ethanol is a solvent used in the pharmaceutical business to make medications, tinctures, and other items.
Industrial applications: Ethanol is utilized as a solvent in the production of several chemical products, such as varnishes, fragrances, and cosmetics.
Scientific Research: Alcohol fermentation (Ethanol) is actively researched to better understand metabolic pathways, enzyme functions, and genetic regulation of microorganisms.
In conclusion, Ethanol fermentation is an important process for many industries, including beverage production, biofuel manufacturing, and biotechnology, because it produces ethanol and CO2 effectively under anaerobic conditions. It is a critical process with numerous uses in industries including beverage production, biofuel manufacturing, biotechnology, food, and medicines. It helps to produce alcoholic beverages, renewable fuels, and a variety of industrial and pharmaceutical items by efficiently converting carbohydrates into ethanol and CO2. Ethanol fermentation’s versatility and efficiency make it critical for sustainable practices and industrial innovation, underlining its continued importance in modern civilization.
Frequently Asked Questions (FAQ)
What organisms are involved in ethanol fermentation?
Yeast, notably Saccharomyces cerevisiae, and some microorganisms produce ethanol.
What are the key steps in ethanol fermentation?
The three primary steps are glycolysis (converting glucose to pyruvate), decarboxylation of pyruvate to acetaldehyde, and reduction of acetaldehyde to ethanol.
What happens to pyruvate during ethanol fermentation?
Pyruvate is decarboxylated to produce acetaldehyde, which is then converted into ethanol.
Related Articles