Affinity Chromatography
Biomolecules can be separated and purified using affinity chromatography, a chromatographic method that is based on the unique interactions that biomolecules have with immobilized ligands. The technique takes use of the strong attraction and focus that exist between a target biomolecule and a ligand that is affixed to a chromatography matrix.
Table of Contents
Definition
Biomolecules can be separated and purified using affinity chromatography, a specialized chromatographic method, according to how well they bind with immobilized ligands. This approach uses a ligand with a high affinity for the target biomolecule in the stationary phase of the chromatographic column. The target attaches to the immobilized ligand selectively when a sample containing the target biomolecule passes through the column, and non-target molecules are washed away. Highly pure and isolated biomolecules can be obtained by eluting the bound biomolecule from the column under circumstances that interfere with the binding relationship. In biochemistry, biotechnology, and pharmaceutical research, affinity chromatography is extensively utilized for the purification of proteins, enzymes, antibodies, and other biomolecules.
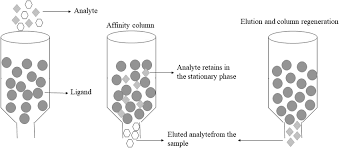
Principle of Affinity Chromatography
1. Ligand Immobilization
The initial stage is immobilizing a particular ligand on a solid substrate, like agarose beads or a membrane. The target molecule that needs to be purified determines which ligand is best. Antibodies, enzymes, receptors, and tiny molecules that bind to the target molecule specifically are examples of common ligands.
2. Application of the Sample
Next, the affinity column or matrix is subjected to the sample containing the combination of biomolecules. Other non-specific molecules either slip through or bind weakly, allowing them to be washed away, but the target molecule will attach selectively to the immobilized ligand.
3. Washing
To further purify the target molecule, any free or weakly bound molecules are removed from the column using a buffer solution after the sample has been applied.
4. Elution
Next, by altering the pH, ionic strength, or temperature to prevent the ligand and target molecule from binding, the target molecule is eluted from the column. This makes it possible to gather the target molecule that has been purified in a concentrated form.
5. Regeneration
In order to free the column from any residual bound molecules and get it ready for more purification cycles, it can be regenerated by washing it with the proper solutions.
Parts of Affinity Chromatography
1. Matrix or supports Material
The solid phase that the ligand is immobilized on is known as the matrix or support material. Membranes, agarose beads, and sepharose beads are examples of common matrix materials. For the binding interactions between the ligand and the target molecule to be supported, the matrix needs to have a high surface area and mechanical stability.
2. Ligand
The molecule that binds to the target molecule of interest specifically is known as the ligand. By covalent linkage or other means, it is immobilized onto the matrix. The target molecule that needs to be purified determines which ligand is best. Small molecules, lectins, enzymes, receptors, and antibodies are examples of common ligands.
3. Sample Application
In this stage, the affinity column or matrix is coated with a sample that contains a combination of biomolecules. Target molecule attaches itself to the immobilized ligand while other non-specific molecules pass through or bind weakly.
4. Wash Buffers
After applying a sample to the column, wash buffers are used to extract molecules that are either free or poorly bound. In order to remove molecules that are not particularly bound to the ligand while leaving the target molecule bound to it, these buffers usually contain detergents and salts.
5. Elution Buffer
Elution buffer prevents the ligand and target molecule from binding together, releasing the purified target molecule from the column. Chaotropic agents, competitive ligands, pH modifiers, and other substances that reduce binding interactions can be found in elution buffers.
6. Collection System
This device used to gather the eluted fractions containing the target molecule that has been purified. Simple gravity flow may be used, while more complex systems like fraction collectors and pumps may be used.
7. Regeneration Buffers
To get rid of any leftover bound molecules and get the column ready for more purification cycles, the column can be regenerated after elution. To get the column back to its initial state so it can be used again, regeneration buffers are utilized.
Steps of Affinity Chromatography
1. Equilibration
A buffer solution that is compatible with the ensuing purification processes is used to equilibrate the affinity column or matrix. The stability and functionality of the ligand and matrix are preserved with the aid of this buffer.
2. Application of the Sample
The affinity column or matrix is coated with the sample that contains the combination of biomolecules. Non-specific molecules either pass through or bind weakly, whereas the target molecule binds to the immobilized ligand in a selective manner.
3. Washing
To get rid of any unattached or weakly bound molecules, wash buffers are used on the column after the sample is applied. In order to remove molecules that are not particularly attached to the ligand while leaving the target molecule bound to it, wash buffers usually consist of detergents and salts.
4. Elution
The target molecule is then eluted from the column by changing the conditions to disrupt the binding between the ligand and the target molecule. Elution buffers are used for this purpose, and they may contain chaotropic agents, competitive ligands, pH modifiers, or other chemicals that weaken the binding interactions. The elution process results in the release of the purified target molecule from the column.
5. Fraction Collection
During elution, fractions containing the purified target molecule are collected in separate tubes or containers. This allows for the collection of the target molecule in a concentrated form for downstream applications.
6. Regeneration
After elution, the column may be regenerated to remove any remaining bound molecules and prepare it for subsequent purification cycles. Regeneration buffers are used to restore the column to its original state for reuse.
Uses of Affinity Chromatography
1. Protein Purification
Based on their unique interactions with immobilized ligands, affinity chromatography is a frequently used method for protein purification. It is a crucial method in protein biochemistry because it makes it possible to isolate proteins with high purity and specificity.
2. Purification of Antibodies
To isolate antibodies from serum, hybridoma culture supernatants, or recombinant expression systems, affinity chromatography is frequently utilized. Protein A/G columns or immobilized antigens can be used to purify antibodies, enabling their use in a variety of research and diagnostic applications.
3. Studies of Receptor-Ligand Interactions
By immobilizing one of the interacting partners on the matrix, affinity chromatography can be utilized to analyze receptor-ligand interactions. This makes it possible to identify and characterize binding partners as well as to determine the affinities and kinetics of binding.
4. Enzyme Purification
By using affinity chromatography, enzymes can be isolated according to the way they interact with inhibitors, cofactors, or analogs of the substrate that have been immobilized on the matrix. This makes it easier to examine structure-function correlations, enzyme kinetics, and biotechnological applications.
5. Purification of Nucleic Acids
Affinity chromatography can be used to separate nucleic acids (DNA, RNA) according to how they specifically interact with binding proteins that are immobilized on the matrix, aptamers, or complementary sequences. It is frequently used in biotechnology and molecular biology for processes including chromatin immunoprecipitation (ChIP), RNA isolation, and plasmid purification.
6. Glycoprotein Purification
Glycoproteins are purified using affinity chromatography according to the particular carbohydrate moieties they contain. Lectins are frequently employed as ligands for the separation of glycoproteins because of their ability to attach to particular carbohydrate structures.
7. Drug Target Identification and Screening
In the process of finding new drugs, affinity chromatography can be used to both identify and describe drug targets as well as screen small molecule libraries for possible candidates. Target proteins or drug-like compounds that have been immobilized can be utilized to locate and recognize interaction partners in complex biological samples.
8. Diagnostic tests

To identify and isolate particular biomarkers or analytes in clinical samples, affinity chromatography is used in diagnostic tests. It makes it possible to isolate target molecules with great sensitivity and specificity, which helps with the creation of diagnostic tests for a range of illnesses.
Frequently Asked Question (FAQ)
What is Affinity Chromatography?
In biochemistry and molecular biology, affinity chromatography is a potent method for separating individual biomolecules from complicated mixtures according to their affinity for a given ligand. The selective and reversible binding of a target molecule (the analyte) to an immobilized ligand on a solid support (the matrix) is the basis for affinity chromatography’s working theory.
What are Applications of Affinity Chromatography
1. Protein purification is the process of separating highly pure proteins.
2. Purification of Antibodies: Isolation of Antibodies for Investigations and Examinations.
3. Enzyme purification is the process of making enzymes more suitable for biotechnological uses and characterization.
4. Purification of Nucleic Acids: Taking out DNA or RNA for use in diagnostics and molecular biology.
5. Glycoprotein Purification: Using carbohydrate moieties as a basis, isolate glycoproteins.
6. Drug Target Identification: The process of locating and defining a drug target.
7. Assays for diagnostic purposes: extraction and identification of biomarkers from clinical samples.
8. Studies of protein-protein interactions look into how different proteins interact with one another.
9. Drug screening is the process of looking for possible drugs by screening small molecule libraries.
10 Biomedical Research: Used to isolate and examine biomolecules in a variety of research fields.
Related Article