In the field of genetics, mapping the position of genes on chromosomes has proven to be one of the most vital tools for understanding heredity and genetic disorders. Chromosome mapping provides a visual representation of gene locations and their distances relative to one another, greatly enhancing our understanding of biological inheritance and disease mechanisms.
With continuous advancements in molecular biology and computational tools, chromosome mapping has evolved from basic linkage analysis to high-resolution, genome-wide sequencing approaches. This article offers a detailed look at the principles, techniques, and applications of chromosome mapping, highlighting its importance in modern biological and medical research.
Summary of Chromosome Mapping
- Chromosome mapping identifies gene locations on chromosomes using genetic and physical methods.
- It helps understand genetic diseases and supports advances in gene therapy and personalized medicine.
- Modern technologies like sequencing and CRISPR have made mapping faster and more accurate.
Table of Contents
Significance of Chromosome Mapping in Modern Genetics
Chromosome mapping holds immense importance in both theoretical and applied genetics. It allows scientists to associate specific regions of DNA with phenotypic traits and genetic diseases, aiding early diagnosis, prognosis, and therapeutic design. Beyond medicine, it contributes significantly to plant and animal breeding programs, forensic science, and biodiversity conservation by identifying key genetic markers.
In modern personalized medicine, chromosome mapping helps tailor treatments based on an individual’s genetic makeup, improving patient outcomes while reducing side effects. In evolutionary studies, it provides valuable insights into species divergence, gene duplication events, and conserved genetic sequences across different organisms.
Historical Background and Milestones
The foundations of chromosome mapping were laid in the early 20th century through experiments on fruit flies (Drosophila melanogaster). Thomas Hunt Morgan’s work on gene linkage earned him the Nobel Prize in 1933, while his student Alfred Sturtevant constructed the first genetic map in 1913. This pioneering work demonstrated that genes are linearly arranged on chromosomes and are inherited together if located nearby.
Over the decades, molecular techniques such as restriction mapping, fluorescence in situ hybridization (FISH), and DNA sequencing further advanced mapping precision. The Human Genome Project, completed in 2003, marked a landmark achievement in physical mapping by sequencing the entire human genome, paving the way for modern genomics.
What is Chromosome Mapping?
Chromosome mapping involves determining the specific locations of genes or markers along a chromosome. It visually represents the arrangement and relative distances between genes, serving as a guide for understanding inheritance patterns, genetic recombination, and genomic organization.
This process is fundamental for identifying genes responsible for inherited disorders, discovering new therapeutic targets, and developing advanced genomic technologies.
Definition of Chromosome Mapping
Chromosome mapping is the scientific technique of assigning specific loci (positions) to genes or genetic markers on chromosomes. These maps help illustrate either the physical position of genes based on DNA sequences or their relative positions determined by recombination frequencies.
Depending on the methodology, chromosome maps can be classified as genetic (linkage) maps or physical maps. Both types serve complementary roles in genetic analysis, genome annotation, and medical research.
The Role of Chromosome Mapping in Genetics
Chromosome maps serve as indispensable tools for connecting genetic sequences with observable traits (phenotypes). By identifying how genes are organized and inherited, these maps help detect disease-associated genes, uncover genetic mechanisms of complex traits, and study gene-environment interactions.
In clinical genetics, chromosome mapping assists in diagnosing chromosomal abnormalities such as deletions, duplications, and translocations. In addition, it informs research on gene regulation, chromosomal behavior during cell division, and epigenetic modifications influencing gene expression.
The Structure of a Chromosome
A fundamental understanding of chromosome anatomy is essential before exploring mapping techniques. Chromosomes are highly organized DNA-protein complexes that package genetic information, ensuring its proper transmission and regulation within the cell.
Chromosomes differ in size, shape, and number among species, and their structural elements influence gene mapping strategies and genomic stability.
Overview of Chromosome Anatomy
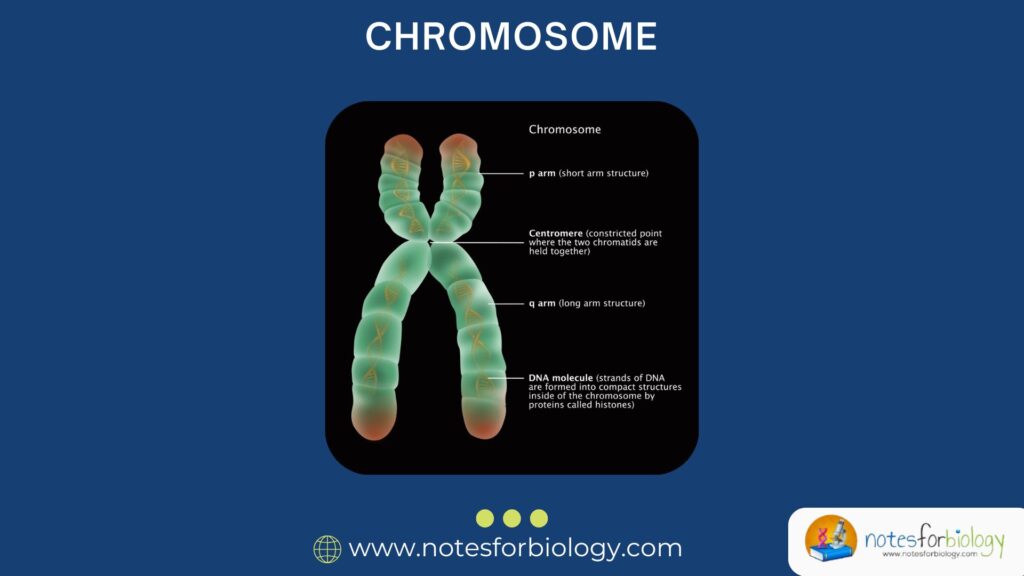
Chromosomes consist of a continuous, linear DNA molecule wrapped around histone proteins, forming a compact chromatin structure. During cell division, chromatin condenses into visible chromosomes, each possessing a centromere that divides it into two arms: a short (p) arm and a long (q) arm.
The ends of chromosomes are protected by telomeres, while regions called origins of replication allow DNA duplication before cell division. The position and behavior of these elements are crucial for maintaining genetic integrity and are considered in chromosome mapping experiments.
Key Terms: Centromere, Telomere, Chromatid
The centromere is a constricted region on a chromosome where spindle fibers attach during cell division, ensuring proper segregation of chromatids. The telomere comprises repetitive DNA sequences at the chromosome ends, preventing degradation and fusion with neighboring chromosomes.
Each duplicated chromosome consists of two identical halves called sister chromatids, which remain connected at the centromere until separated during anaphase. Recognizing these parts is vital for cytogenetic mapping and understanding chromosomal behavior in genetic diseases.
Types of Chromosome Mapping
Chromosome mapping strategies are categorized based on the data source and the kind of information they reveal about the genome. Three major types—genetic (linkage) mapping, physical mapping, and comparative mapping are widely used, each with distinct methodologies and applications.
By integrating these approaches, researchers can construct high-resolution maps covering both the genetic and physical landscapes of a genome.
Genetic (Linkage) Mapping
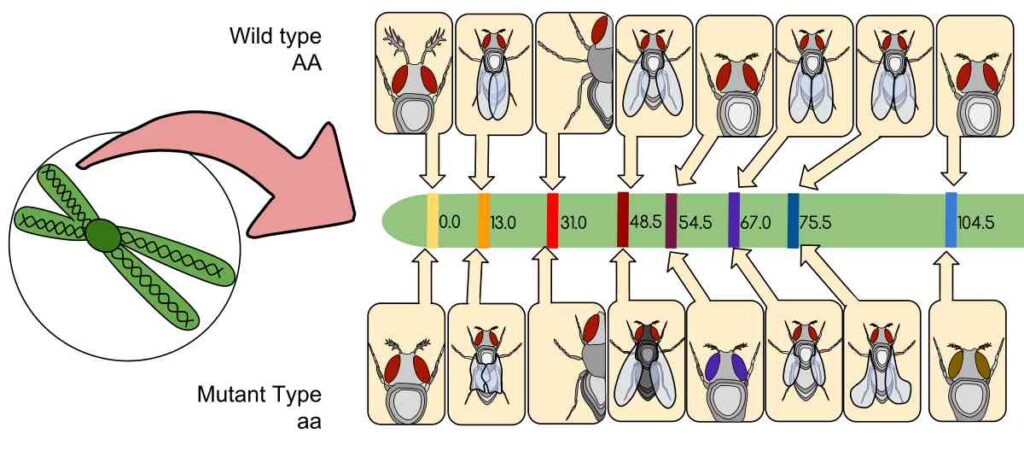
Genetic mapping estimates the relative positions of genes on a chromosome based on the frequency of recombination during meiosis. Genes closer together tend to be inherited together, while those farther apart undergo recombination more frequently.
Definition and Methodology
A genetic map is constructed by analyzing inheritance patterns within families or experimental crosses and calculating the recombination frequencies between markers. These frequencies, expressed in centimorgans (cM), reflect the likelihood of crossover events separating two loci during meiosis.
By compiling recombination data across multiple generations, scientists establish the linear order and approximate genetic distances between genes.
Historical Achievements
The concept of genetic mapping was first applied to fruit flies by Alfred Sturtevant, whose 1913 map demonstrated the linear arrangement of genes. Later, linkage studies in humans identified genes associated with diseases like cystic fibrosis, Huntington’s disease, and muscular dystrophy.
Modern linkage mapping has expanded to include microsatellites, single nucleotide polymorphisms (SNPs), and whole-genome association studies, greatly enhancing the resolution and applicability of genetic maps.
Physical Mapping
Physical mapping determines the exact physical locations of genes or markers on chromosomes, typically measured in base pairs (bp). Unlike linkage mapping, it involves direct examination of the DNA molecule or chromosomal structure.
Definition and Techniques
Physical maps are created using laboratory methods such as restriction fragment analysis, FISH, radiation hybrid mapping, and whole-genome sequencing. These techniques provide precise measurements of DNA distances and positions, enabling the identification of mutations, gene clusters, and structural variations.
In practice, physical maps are indispensable for sequencing projects, clinical diagnostics, and chromosomal rearrangement studies.
Differences from Genetic Mapping
While genetic maps reflect the relative positions of genes based on inheritance patterns, physical maps provide absolute positional data. Physical mapping is particularly useful for identifying genes in regions of low recombination and for confirming findings from linkage analyses.
Moreover, physical mapping resolves structural genome complexities, including duplications, deletions, and inversions, which are challenging to detect through recombination-based methods.
Comparative Mapping
Comparative mapping involves aligning chromosome maps from different species to identify conserved genetic regions and investigate evolutionary relationships. It’s a powerful approach in comparative genomics and functional gene annotation.
Definition and Applications
By comparing gene order and location across species, comparative mapping identifies evolutionary conserved segments known as synteny blocks. These regions reveal insights into the conservation of gene function and genome evolution.
Applications include tracing the ancestry of gene families, transferring knowledge from model organisms to humans, and optimizing breeding programs by identifying shared genetic markers in livestock or crops.
Techniques Used in Chromosome Mapping
To accurately construct chromosome maps, various specialized techniques have been developed, each suited to different research goals and levels of resolution. These methods range from classical genetic analyses to advanced molecular cytogenetics and high-throughput sequencing technologies.
A comprehensive understanding of these techniques is crucial for selecting appropriate approaches in genetic research, clinical diagnostics, and genomic studies.
Linkage Analysis
Linkage analysis is one of the oldest and most widely used methods in genetic mapping. It involves studying how genes are inherited together within families or populations and determining the likelihood of recombination between them.
This technique is especially valuable in locating genes responsible for inherited diseases and understanding patterns of inheritance in both humans and model organisms.
Linkage analysis typically requires pedigree data and genotyping of multiple family members across generations. Statistical algorithms are then applied to estimate recombination frequencies, which help establish the relative positions of genes on a genetic map.
Fluorescence In Situ Hybridization (FISH)
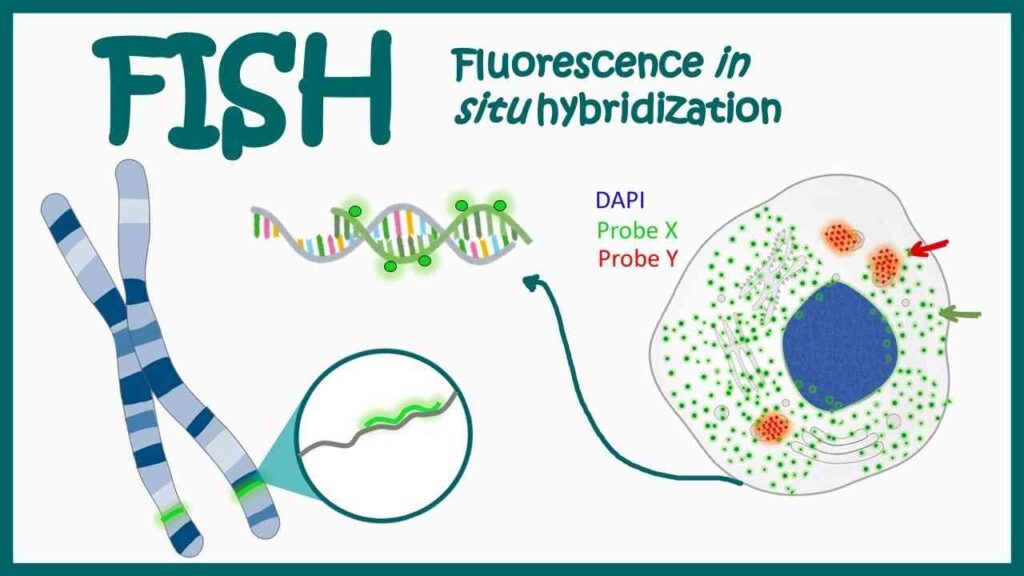
FISH is a molecular cytogenetic technique that uses fluorescently labeled DNA probes to detect specific DNA sequences directly on chromosomes. Under a fluorescence microscope, these probes bind to their complementary sequences, emitting a signal that reveals the precise chromosomal location.
FISH is commonly used to identify chromosomal abnormalities such as deletions, duplications, and translocations, especially in cancer cytogenetics and prenatal diagnostics.
Its advantages include the ability to visualize chromosomes in their natural state within the nucleus and detect structural changes that might not be identified by traditional karyotyping. Multiple probes can be used simultaneously (multiplex FISH) for complex chromosomal analyses.
Radiation Hybrid Mapping
Radiation hybrid (RH) mapping involves exposing cells to ionizing radiation to fragment chromosomes and then fusing these cells with recipient cells to create hybrid lines. By analyzing which genetic markers are retained together in hybrid cells, scientists can estimate the physical proximity of genes.
This technique bridges the gap between genetic and physical mapping and is particularly useful for building high-density maps of complex genomes.
RH mapping offers moderate resolution and helps to order DNA markers along chromosomes, especially in species where sequencing data is incomplete. It has been employed in mapping human, mouse, and livestock genomes.
DNA Sequencing and Bioinformatics Tools
Advances in DNA sequencing technologies have revolutionized chromosome mapping, enabling precise, high-throughput identification of gene locations and sequence variations. Modern techniques include whole-genome sequencing (WGS), targeted sequencing, and long-read platforms like nanopore and PacBio sequencing.
Bioinformatics tools complement sequencing by aligning reads, assembling genomes, and annotating gene positions, regulatory elements, and structural variants.
These combined approaches allow for comprehensive physical mapping and are integral to projects like the Human Genome Project and modern personalized medicine initiatives. They also aid in discovering novel mutations and understanding genome-wide associations with traits and diseases.
Importance of Chromosome Mapping
The importance of chromosome mapping extends far beyond academic research. It plays a central role in clinical genetics, agricultural improvements, evolutionary biology, and pharmaceutical development.
By linking specific genes to traits and disorders, chromosome mapping guides targeted interventions, facilitates early diagnosis, and supports predictive and personalized approaches to medicine and healthcare.
Understanding Genetic Disorders
Chromosome mapping has been instrumental in identifying the genetic basis of numerous inherited diseases. By pinpointing the exact location of defective genes, scientists can develop diagnostic tests, genetic screenings, and targeted therapies.
This knowledge allows for prenatal testing, carrier detection, and risk assessment in families with a history of genetic disorders such as cystic fibrosis, sickle cell anemia, and hemophilia.
Furthermore, mapping helps in understanding polygenic diseases, where multiple genes contribute to conditions like diabetes, hypertension, and psychiatric disorders, aiding in developing comprehensive treatment strategies.
Gene Localization and Identification
Accurate localization of genes on chromosomes provides insights into gene function, interactions, and regulatory mechanisms. This is essential for studying complex traits influenced by multiple genetic and environmental factors.
Gene localization also assists in identifying regulatory elements such as enhancers, silencers, and promoters that control gene expression. Understanding these elements contributes to fields like epigenetics and developmental biology.
In practical applications, this knowledge helps scientists design gene therapies, functional genomics studies, and synthetic biology constructs with precision.
Advancements in Gene Therapy
Gene therapy involves replacing, repairing, or regulating defective genes to treat genetic diseases. Chromosome mapping guides these interventions by identifying safe integration sites and target loci within the genome.
With precise mapping data, researchers can avoid disrupting essential genes and minimize the risk of insertional mutagenesis. This has been critical in the development of gene therapies for spinal muscular atrophy (SMA), inherited blindness, and certain immunodeficiency disorders.
Emerging gene-editing technologies like CRISPR-Cas9 rely heavily on accurate chromosome maps to target disease-causing mutations precisely and safely.
Agricultural and Plant Genome Studies
In agriculture, chromosome mapping is invaluable for identifying genes associated with desirable traits such as drought tolerance, pest resistance, and high yield. This information accelerates crop improvement programs through marker-assisted selection and genetic engineering.
Mapping also aids in conserving plant genetic diversity and tracing the domestication and evolution of crop species. Similar applications exist in animal breeding, where mapping helps identify markers linked to disease resistance, productivity, and desirable phenotypes.
These advancements contribute to global food security, sustainable farming practices, and the development of climate-resilient agricultural systems.
Applications in Medicine and Research
Chromosome mapping is a foundational tool in medical genetics and biomedical research. By identifying specific genes and their positions, it enables the understanding of disease mechanisms, improves diagnostics, and facilitates the development of precision treatments.
Its applications extend to various domains such as oncology, pharmacogenomics, reproductive medicine, and evolutionary research, thereby integrating genomics into multiple disciplines.
Personalized Medicine and Pharmacogenomics
Chromosome mapping plays a crucial role in the emergence of personalized medicine, where treatments and drug regimens are tailored to an individual’s genetic profile. By identifying genetic variations in drug metabolism genes, clinicians can predict how a patient will respond to certain medications.
Pharmacogenomics uses this mapping data to minimize adverse drug reactions and maximize therapeutic efficacy. For instance, variations in the CYP450 gene family influence how individuals metabolize common drugs, guiding dosage adjustments.
Moreover, mapping helps detect genes associated with predisposition to diseases, enabling early interventions and personalized health plans aimed at disease prevention and longevity.
Cancer Genomics and Cytogenetics
In cancer biology, chromosome mapping is essential for detecting oncogenes, tumor suppressor genes, and chromosomal abnormalities associated with malignancy. Mapping techniques like FISH, CGH (Comparative Genomic Hybridization), and sequencing allow identification of gene amplifications, deletions, and translocations.
These findings guide the development of targeted therapies such as HER2 inhibitors in breast cancer or BCR-ABL inhibitors in chronic myeloid leukemia. Furthermore, mapping supports prognosis by identifying chromosomal markers linked to aggressive tumor behavior or therapeutic resistance.
Cytogenetic profiling of tumors through mapping has become a routine part of modern cancer diagnostics, leading to more informed treatment strategies and patient-specific care.
Evolutionary Biology and Species Comparison
Chromosome mapping is a powerful comparative tool in evolutionary biology. By aligning genetic maps across different organisms, researchers can identify conserved gene blocks and trace lineage-specific chromosomal rearrangements.
These comparative maps help in reconstructing evolutionary histories, estimating divergence times, and understanding genome organization patterns across taxa. For example, similarities between human and chimpanzee chromosome maps reveal shared ancestry and key differences in gene regulation.
Such insights not only improve our understanding of evolutionary biology but also enhance the use of model organisms in biomedical research by identifying homologous genes and conserved pathways.
Recent Advances in Chromosome Mapping
With the advent of advanced molecular biology and computational tools, chromosome mapping has seen significant technological improvements. These innovations have enhanced the speed, accuracy, and scope of mapping, enabling whole-genome analyses at an unprecedented resolution.
Newer techniques have facilitated the identification of complex genomic rearrangements, structural variants, and rare mutations that were previously undetectable.
Next-Generation Sequencing (NGS)
NGS technologies have revolutionized chromosome mapping by allowing millions of DNA fragments to be sequenced in parallel. This provides a high-resolution overview of the genome, enabling researchers to map not only gene locations but also regulatory elements and structural changes.
NGS has applications in both research and clinical diagnostics, offering faster turnaround times and lower costs compared to traditional Sanger sequencing. It supports whole-exome, whole-genome, and targeted panel sequencing, tailored to various research and clinical needs.
Additionally, NGS integrates well with bioinformatics tools for variant calling, gene annotation, and comparative genomics, forming the backbone of modern genomic studies.
CRISPR-based Mapping Approaches
CRISPR-Cas systems have emerged as precise and versatile tools for genome editing and mapping. Researchers now use CRISPR to label specific DNA sequences in live cells, enabling real-time observation of chromosomal loci and gene regulation events.
CRISPR-mediated chromatin imaging and epigenome editing provide novel insights into chromosomal architecture and gene-environment interactions. These mapping applications are particularly valuable for studying dynamic genome organization in response to environmental stimuli or developmental changes.
Moreover, CRISPR enhances functional mapping by allowing researchers to systematically knock out genes and observe phenotypic outcomes, helping assign functions to previously uncharacterized genomic regions.
Whole Genome Mapping Technologies
New platforms such as optical mapping, nanochannel arrays, and long-read sequencing (PacBio, Oxford Nanopore) have significantly improved our ability to resolve structural complexities in genomes. These methods provide long contiguous DNA reads, ideal for spanning repetitive regions and large rearrangements.
Whole-genome mapping allows for the detection of copy number variations, large insertions or deletions, and chromosomal fusions that are often missed by short-read technologies. These technologies are particularly valuable in cancer genomics, rare disease discovery, and microbial genome assembly.
By integrating long-range information with high-resolution data, whole-genome mapping brings us closer to complete, gapless reference genomes for many species.
Challenges in Chromosome Mapping
Despite its power and utility, chromosome mapping still faces several technical, biological, and ethical challenges. These limitations can affect the accuracy of mapping, interpretation of results, and societal acceptance of genomic technologies.
Overcoming these barriers requires ongoing innovation, improved data analysis tools, and robust regulatory frameworks for ethical practice.
Complexity of Eukaryotic Genomes
Eukaryotic genomes are large, complex, and rich in repetitive sequences, making it difficult to assemble and accurately map certain regions. Highly repetitive elements, such as satellite DNA and transposons, often lead to sequencing errors and gaps in genome assemblies.
In addition, alternative splicing, gene duplication, and regulatory elements located far from coding genes complicate the interpretation of map data. These complexities necessitate a combination of physical mapping, long-read sequencing, and chromatin conformation studies for comprehensive genome analysis.
Accurate annotation and functional validation are crucial to derive meaningful biological insights from chromosome maps, especially in non-model organisms.
Ethical and Privacy Concerns in Genetic Data
As chromosome mapping becomes more integrated into healthcare and population studies, ethical issues related to privacy, data ownership, and potential misuse of genetic information come to the forefront. Individuals may fear genetic discrimination in employment or insurance based on their genomic profiles.
There are also concerns about informed consent, particularly when data is shared across institutions or countries. Genome-wide association studies and biobank research require transparent communication, anonymization safeguards, and community involvement.
Addressing these issues is critical for public trust, responsible science, and equitable access to the benefits of genomic medicine.
Future Prospects of Chromosome Mapping
The future of chromosome mapping is poised to transform not only biological research but also healthcare, agriculture, and environmental sciences. Emerging technologies and integrative data approaches are expanding the possibilities of what mapping can achieve.
As we move toward precision biology, chromosome mapping will play an increasingly central role in predictive, preventive, and participatory models of science and medicine.
Predictive Genomics
By combining chromosome maps with risk allele data and environmental factors, predictive genomics aims to forecast an individual’s likelihood of developing certain diseases. This has immense potential in preventing chronic diseases like cancer, diabetes, and cardiovascular conditions through early lifestyle and medical interventions.
Predictive tools based on chromosome mapping can also aid in assessing inherited disease risk, improving reproductive decision-making, and optimizing treatment pathways. The future may see the integration of personal genome maps into routine health check-ups and digital health platforms.
Precision Agriculture and Livestock Breeding
Chromosome mapping will continue to revolutionize agriculture by enabling breeders to select for traits such as drought resistance, pest tolerance, improved nutritional content, and reproductive efficiency. Marker-assisted selection and genome editing depend heavily on accurate maps for success.
In livestock breeding, mapping supports the identification of genes associated with milk yield, disease resistance, fertility, and meat quality. Genomic selection based on mapping data enhances productivity while maintaining genetic diversity and animal welfare.
This contributes to sustainable food systems, increased resilience to climate change, and better economic outcomes for farmers.
Potential in Rare Disease Diagnosis
Rare diseases often go undiagnosed due to their complexity and lack of data. Chromosome mapping, especially when integrated with NGS, enables the discovery of novel genes and mutations responsible for such conditions.
Initiatives like the 100,000 Genomes Project and Undiagnosed Diseases Network use chromosome mapping to solve diagnostic mysteries, providing answers to families and new opportunities for research and therapy development.
Mapping will also assist in stratifying rare diseases into subtypes based on genetic cause, improving the precision and relevance of future treatments.
Conclusion
Chromosome mapping remains one of the most powerful and versatile tools in modern genetics. It provides the foundation for understanding the organization, function, and evolution of genomes, and its applications span health, agriculture, and basic science.
As technologies advance, chromosome maps are becoming more precise, interactive, and integrated with functional data. They will continue to guide discoveries in gene therapy, cancer treatment, personalized medicine, and sustainable food production.
Looking ahead, chromosome mapping will play a central role in shaping the future of science and society, offering new possibilities for improving human health, conserving biodiversity, and solving global challenges through genomics.
Frequently Asked Questions (FAQ)
What is the primary difference between genetic and physical mapping?
Genetic mapping estimates the relative positions of genes based on recombination frequencies during inheritance, while physical mapping determines the exact physical locations of genes on chromosomes using DNA-based techniques.
How is chromosome mapping useful in agriculture?
Chromosome mapping helps identify genes linked to desirable traits like disease resistance and drought tolerance, enabling faster and more precise crop and livestock breeding through marker-assisted selection.
What techniques are most accurate for chromosome mapping?
Techniques like whole-genome sequencing, fluorescence in situ hybridization (FISH), and long-read sequencing (e.g., PacBio, Oxford Nanopore) provide the highest accuracy in chromosome mapping.
Related Contents
Chromatography: Principles, Techniques, and Applications
Cholesterol Structure & Synthesis: Pathways, Functions, and Clinical Importance