Chlorophyll is one of the most vital pigments in the natural world, recognized for its essential role in photosynthesis , the process through which plants, algae, and certain bacteria convert light energy into chemical energy. Beyond its ecological significance, this green pigment holds considerable value in fields such as human nutrition, pharmaceuticals, industrial applications, and environmental science.
This article offers a comprehensive exploration of this pigment, from its structure and types to its biological functions, synthesis process, and modern uses. It will also discuss recent research directions, highlighting its growing importance in sustainable technologies and biotechnology.
Table of Contents
Importance in Plant Biology and Environmental Science
This pigment is indispensable in plant biology because it captures light energy required for photosynthesis, enabling the production of organic compounds that support the food chain. It also plays a key role in maintaining the global carbon and oxygen cycles.
In environmental science, pigment levels serve as indicators of plant health, ecosystem productivity, and the presence of algal blooms. Its reflective and light-absorbing properties make it valuable in ecological assessments and remote sensing applications.
Brief Overview of Biological and Industrial Relevance
In biological systems, this green pigment supports nearly all life on Earth by allowing autotrophic organisms to convert inorganic substances into energy-rich organic molecules. Its benefits extend to human health through antioxidant, detoxifying, and wound-healing effects.
Industrially, it is used as a natural colorant in foods, cosmetics, and emerging sustainable materials. Advances in biotechnology have introduced new possibilities for this pigment in renewable energy, medicine, and eco-friendly product manufacturing.
What is Chlorophyll?
Understanding its broad applications starts with defining this pigment and explaining its critical biological role. It drives photosynthesis while also influencing a range of plant physiological processes.
Definition
This natural pigment is a green, light-absorbing molecule found in plants, algae, and cyanobacteria. It captures solar energy, enabling organisms to convert carbon dioxide and water into glucose and oxygen the foundation of autotrophic nutrition.
The distinctive green hue results from its ability to absorb red and blue wavelengths while reflecting green light. Several forms of this pigment exist, each with unique absorption properties and ecological roles.
Role in Photosynthesis
Photosynthesis occurs in the chloroplasts of plant cells, where pigment molecules are embedded in thylakoid membranes. Upon absorbing light, these molecules transfer energy to reaction center proteins, triggering electron transport and the formation of ATP and NADPH.
These energy carriers drive the Calvin cycle, fixing carbon dioxide into carbohydrates. Without this pigment, photosynthesis — responsible for the planet’s oxygen supply and food production would not be possible.
Chemical Structure
The unique molecular structure of this pigment allows it to efficiently capture light and participate in electron transfer processes. Understanding this structure helps clarify its role in photosynthesis.
Basic Molecular Structure
Molecules of this pigment consist of a porphyrin ring system with alternating double and single bonds, attached to a long phytol chain that anchors them into the thylakoid membrane. The conjugated ring system permits the delocalization of electrons, which is essential for absorbing light energy.
Porphyrin Ring and Central Magnesium Atom
At the heart of the porphyrin ring is a central magnesium ion, which stabilizes the molecule and facilitates light absorption. This arrangement enables efficient capture of specific wavelengths in the visible spectrum, particularly in the red and blue regions.
Structural Differences Among Types
Although the pigment’s basic structure is similar across forms, variations in side chains create distinct types with different absorption spectra. These differences allow photosynthetic organisms to adapt to diverse light conditions in their environments.
Types of Chlorophyll
This pigment occurs in several distinct forms, each suited to specific organisms and habitats. Their structural differences and light absorption properties enable efficient photosynthesis under varied environmental conditions.
Chlorophyll a
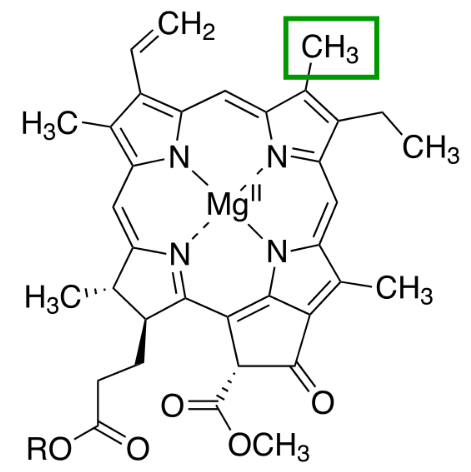
Chlorophyll a is the primary pigment in all oxygenic photosynthetic organisms. It initiates photochemical reactions by transferring excited electrons to the electron transport chain.
Absorbing light most efficiently in the blue-violet and red regions, it is indispensable for photosynthesis.
Chlorophyll b
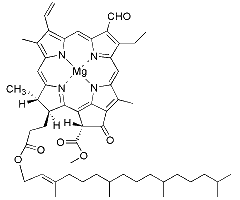
An accessory pigment in green plants and algae, chlorophyll b extends the range of light wavelengths captured for photosynthesis. It mainly absorbs blue and orange light, transferring the captured energy to chlorophyll a.
This improves photosynthetic efficiency, especially in shaded environments.
Chlorophyll c, d, and f
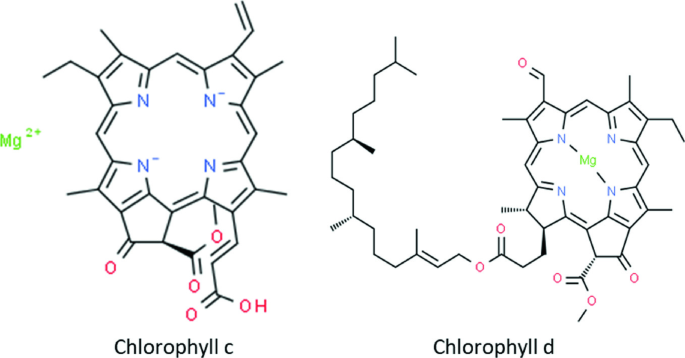
Chlorophyll c is present in certain algae like diatoms, while chlorophyll d and f are found in some cyanobacteria. These pigments absorb different light wavelengths, enabling photosynthesis in environments with limited or filtered light.
Chlorophyll f, discovered relatively recently, is particularly efficient at absorbing far-red light.
Distribution and Unique Features
The distribution of pigment types reflects evolutionary adaptations to ecological niches. Aquatic environments, shaded forests, and extreme habitats often contain specialized pigment variants, maximizing photosynthetic capacity under varied conditions.
Biosynthesis
The production of this pigment is a multi-step, enzyme-driven process occurring primarily in plant plastids. It transforms simple precursors into the complex chlorophyll structure, concluding with magnesium insertion.
Biosynthetic Pathway Overview
The process starts with 5-aminolevulinic acid (ALA), progressing through several stages to form protoporphyrin IX, the precursor to both heme and this pigment. Magnesium is inserted into protoporphyrin IX, followed by modifications that attach the phytol tail.
This pathway is tightly regulated to balance pigment production with environmental and cellular needs.
Key Enzymes
Several enzymes catalyze steps in this pathway, including ALA dehydratase, porphobilinogen deaminase, and magnesium chelatase. Magnesium chelatase directs the metabolic branch toward pigment synthesis rather than heme.
Regulation of Biosynthesis
The biosynthesis is influenced by light intensity, photoperiod, and nutrient availability. Feedback mechanisms adjust enzyme activity, preventing the buildup of toxic intermediates and aligning pigment production with photosynthetic demands.
Factors Affecting Content in Plants
The amount of this pigment in plant tissues varies based on internal and external factors. These changes affect photosynthetic capacity, growth, and ecological adaptability.
Light Intensity and Quality
Light is the primary regulator of pigment production. Higher light levels generally boost synthesis, while shaded or low-light conditions reduce pigment content. The ratio of red to far-red light also influences biosynthesis.
Soil Nutrients
Nutrients like nitrogen, magnesium, and iron are essential for pigment formation. Deficiencies cause chlorosis, a yellowing of leaves due to reduced pigment levels.
Effective soil and fertilizer management directly impacts pigment concentrations.
Water Stress and Environmental Factors
Drought, heat, salinity, and pollution stress plants, inhibiting pigment biosynthesis and accelerating its degradation. These stresses reduce photosynthesis and crop yields.
Monitoring pigment levels assists in assessing plant health and managing stress responses.
Methods to Measure Content
Quantifying this pigment helps evaluate plant health and photosynthetic performance. Techniques range from quick field tools to precise laboratory assays.
SPAD Meter
A SPAD meter provides instant, non-destructive pigment readings by measuring leaf transmittance. It’s widely used in agriculture for crop monitoring, nutrient management, and detecting early stress symptoms.
Spectrophotometric Assays
This lab technique extracts pigment from leaves and measures absorbance at specific wavelengths to calculate pigment concentrations. It remains a research standard due to its precision.
Fluorescence Analysis
Chlorophyll fluorescence assesses photosystem II efficiency and indirectly estimates pigment content. It’s popular in plant physiology research and crop improvement studies.
Plant Health and Photosynthesis
Pigment levels directly reflect plant health and photosynthetic capacity. Measuring content aids in diagnosing nutrient issues and environmental stress.
Photosynthetic Efficiency
Higher pigment levels improve light capture and energy conversion. Deficiencies impair photosynthesis, reducing plant productivity and yield.
Early Stress Detection
Declining pigment content precedes visible damage, offering early warning for issues like drought, pests, or pollution. Early intervention enhances plant resilience.
Health and Therapeutic Uses
This pigment has attracted interest in nutrition and alternative medicine for its biological benefits. It’s used in supplements, skincare, and wound care.
Human Nutrition
Abundant in leafy greens, it contributes antioxidants and supports detoxification. While it adds little caloric value, its health-promoting effects are noteworthy.
Antioxidant Properties
The pigment neutralizes reactive oxygen species and may bind to toxins, aiding detoxification and reducing oxidative stress.
Wound Healing
Topical applications promote healing and odor control. Chlorophyllin, a water-soluble form, is valued for antimicrobial and anti-inflammatory effects.
Industrial Applications
Natural, biodegradable, and safe, this pigment finds use in multiple industries. It replaces synthetic chemicals in food, cosmetics, and materials.
Food Coloring
Approved as a natural colorant, it adds green hues to beverages, candies, and dairy products. Its antioxidant nature enhances product shelf life.
Cosmetics
This pigment’s soothing and antimicrobial effects make it a sought-after ingredient in skincare and hygiene products.
Sustainable Materials
Research explores its use in biodegradable plastics and UV-resistant packaging, aligning with eco-friendly initiatives.
Environmental Science Applications
Chlorophyll content in aquatic and terrestrial ecosystems serves as a critical indicator of primary productivity, nutrient dynamics, and ecological balance. Environmental monitoring programs routinely measure chlorophyll to assess water quality and vegetation health.
Chlorophyll’s properties also enable its use in emerging biotechnological and environmental remediation applications.
Use in Algae Biofuel Production
Microalgae, rich in chlorophyll, are cultivated as renewable biofuel sources. The pigment’s photosynthetic capacity allows microalgae to convert sunlight and carbon dioxide into lipids and biomass, which can be processed into biodiesel, bioethanol, and biogas.
Algae-based biofuels offer a sustainable alternative to fossil fuels and contribute to carbon sequestration.
Applications in Wastewater Treatment
Chlorophyll-containing microalgae are employed in wastewater treatment systems to remove nutrients, heavy metals, and pollutants. Through photosynthesis, these algae assimilate nitrogen and phosphorus, reducing eutrophication risks in water bodies.
Their biomass can subsequently be harvested for bioenergy production or converted into valuable by-products like fertilizers and animal feed.
Recent Advances in Chlorophyll Research
Ongoing research continues to reveal new insights into chlorophyll’s functions and potential applications. Advances in molecular biology, genetic engineering, and material science are expanding its utility beyond traditional roles.
Genetic Engineering for Enhanced Chlorophyll Production
Scientists are using genetic modification techniques to boost chlorophyll content and photosynthetic efficiency in crops. By manipulating genes involved in chlorophyll biosynthesis and degradation, researchers aim to develop plants with higher productivity, stress tolerance, and carbon fixation capacity.
Such innovations hold promise for improving food security and mitigating climate change impacts.
Synthetic Chlorophyll Analogues
Chemists are developing synthetic chlorophyll analogues with tailored properties for applications in photodynamic therapy, solar energy harvesting, and smart materials. These compounds mimic the light-absorbing capabilities of natural chlorophyll while offering enhanced stability and functional versatility.
They are poised to contribute to advances in medicine, renewable energy, and nanotechnology.
Conclusion
Chlorophyll remains a cornerstone of life on Earth, sustaining ecosystems through its central role in photosynthesis. Its functions extend far beyond plant physiology, influencing environmental health, human nutrition, medicine, and industry.
As scientific understanding deepens and biotechnological tools advance, chlorophyll’s potential applications continue to expand. Future research will likely focus on enhancing photosynthetic efficiency, developing eco-friendly materials, and harnessing chlorophyll derivatives for medical and industrial innovations.
Frequently Asked Questions (FAQ)
Why is chlorophyll essential for plant survival?
Chlorophyll is crucial for plants because it captures sunlight energy and drives photosynthesis, allowing plants to produce food (glucose) and release oxygen necessary for life.
How does chlorophyll help in detoxifying the human body?
Chlorophyll binds to certain toxins, heavy metals, and harmful compounds in the body, aiding their removal and reducing oxidative stress through its antioxidant properties.
Which plants have the highest chlorophyll content?
Dark green leafy vegetables like spinach, parsley, wheatgrass, kale, and chlorella algae have some of the highest natural chlorophyll concentrations.
Related Contents
Chiasmata- Definition, Formation, Structure, Significances
Chemical Reactions: Types, Balancing, Influencing Factors, and Reaction Rates